The Project Gutenberg eBook of Natural History of Cottonmouth Moccasin, Agkistrodon piscovorus (Reptilia)
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: Natural History of Cottonmouth Moccasin, Agkistrodon piscovorus (Reptilia)
Author: Ray D. Burkett
Release date: December 15, 2011 [eBook #38308]
Language: English
Credits: Produced by Chris Curnow, Joseph Cooper and the Online
Distributed Proofreading Team at https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK NATURAL HISTORY OF COTTONMOUTH MOCCASIN, AGKISTRODON PISCOVORUS (REPTILIA) ***
University of Kansas Publications
Museum of Natural History
Vol. 17, No. 9, pp. 435-491, 7 figures in text
 October 27, 1966
October 27, 1966 
Natural History of Cottonmouth Moccasin,
Agkistrodon piscivorus (Reptilia)
BY
RAY D. BURKETT
University of Kansas
Lawrence
1966
[436]
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, Henry S. Fitch,
Frank B. Cross
Vol. 17, No. 9, pp. 435-491, 7 figures in text
Published
October 27, 1966
University of Kansas
Lawrence, Kansas
PRINTED BY
ROBERT R. (BOB) SANDERS, STATE PRINTER
TOPEKA, KANSAS
1966
31-4629
[437]
Natural History of Cottonmouth Moccasin,
Agkistrodon piscivorus (Reptilia)
BY
RAY D. BURKETT
CONTENTS
| PAGE | ||
| Introduction | 439 | |
| Acknowledgments | 440 | |
| Systematic Relationships and Distribution | 441 | |
| Description | 444 | |
| Color and Pattern | 444 | |
| Scutellation | 444 | |
| Dentition | 449 | |
| Habitat and Limiting Factors | 450 | |
| Reproduction | 452 | |
| Courtship and Mating | 452 | |
| Reproductive Cycles | 452 | |
| Embryonic Development | 454 | |
| Birth of Young | 454 | |
| Number of Young per Litter | 454 | |
| Population Composition | 455 | |
| Reproductive Potential | 455 | |
| Growth and Development | 456 | |
| Size at Birth and Early Growth | 456 | |
| The Umbilical Scar | 457 | |
| Later Growth and Bodily Proportions | 457 | |
| Shedding | 459 | |
| The Shedding Operation | 459 | |
| Frequency of Shedding | 460 | |
| Food Habits | 461 | |
| Methods of Obtaining Prey | 461 | |
| Food and Food Preferences | 462 | |
| Mortality Factors | 465 | |
| Natural Enemies and Predators | 465 | |
| Parasites and Diseases | 465 | |
| Miscellaneous Causes of Death | 466 | |
| Behavior | 466 | |
| Annual and Diel Cycles of Activity | 466 | |
| Basking | 469 | |
| Coiling | 469 | |
| Locomotion | 470 | |
| Disposition | 470 | |
| Defense and Escape | 471 | |
| "Head Bobbing" | 471 | |
| Combat Dance | 472 | |
| The Venom | 473 | |
| Properties of the Venom | 473 | |
| Venom Yield and Toxicity | 473 | |
| Susceptibility of Snakes | 475 | |
| The Bite | 476 | |
| Effects of the Bite | 476 | |
| Treatment | 477 | |
| Case History of a Bite | 479 | |
| Snakebite in the United States | 480 | |
| Summary | 480 | |
| Literature Cited | 485 | |
[439]
INTRODUCTION
Objectives of the study here reported on were to: (1) learn as
much as possible concerning the natural history and economic
importance of the cottonmouth; (2) determine what factors limit
its geographic distribution; (3) determine the role of the cottonmouth
in its ecological community; and (4) compare the cottonmouth's
life history with that of other crotalid snakes, especially the
kinds that are most closely related to it.
Twenty-five live cottonmouths were kept in the laboratory for
the purpose of studying behavior and fang shedding and for comparison
of measurements with those of preserved specimens. Live
snakes were obtained in Brazoria and Nacogdoches counties, Texas,
from Hermann Park Zoo, Houston, Texas, and from the late Paul
Anderson of Independence, Missouri. Preserved western cottonmouths
were examined for the purpose of determining variation,
distribution, food habits, body proportions, embryonic development,
and reproductive cycles. The cottonmouths examined include:
221 from Texas; 33 from Arkansas; 22 from Louisiana; 2
from Illinois; and 1 each from Kansas, Mississippi, and Oklahoma.
In the preparation of this report I have examined all available
literature pertaining to the cottonmouth and have drawn from
these sources for comparative or additional material. Some of the
more noteworthy contributions to knowledge of the cottonmouth
are the general accounts of the life history by Allen and Swindell
(1948), Barbour (1956), and Wright and Wright (1957); the publications
by Gloyd and Conant (1943) concerning taxonomy;
Klimstra (1959) concerning food habits; and Allen (1937), Parrish
and Pollard (1959), Swanson (1946), and Wolff and Githens
(1939b) concerning the venom. Numerous other publications,
although brief, contain worthwhile contributions. Also of special
interest as a source of material for comparison of cottonmouths with
other crotalids are the works of Fitch (1960) on the copperhead and
of Klauber (1956) on the rattlesnakes.
The cottonmouth has been well known for nearly 200 years.
Wright and Wright (1957) listed the following vernacular names
that are applied to the cottonmouth: black moccasin, black snake,
blunt-tail moccasin, congo, copperhead, cottonmouth water moccasin,
cotton-mouthed snake, gapper, highland moccasin, lowland
moccasin, mangrove rattler, moccasin, North American cottonmouth
snake, North American water moccasin, North American
water viper, pilot, rusty moccasin, salt-water rattler, stubtail, stump [440] (-tail) moccasin, stump-tail viper, swamp lion, Texas Moccasin,
trapjaw, Troost's moccasin, true horn snake, true water moccasin,
viper, water mokeson, water pilot, water rattlesnake, and water
viper.
Some of the names listed above are based upon superstition and
folklore prevailing in pioneer times, and others are based upon the
behavior or appearance of the snake at various ages. Names like
"stump-tail moccasin" are derived from the appearance of females
which have short tails or snakes that have lost part of the tail.
Names like "gapper" and "trapjaw" came to be applied because
of the habit of the snake's lying with its mouth open when approached.
The name "cottonmouth" also was derived from this
behavior, although the lining of the mouth is whitish in most other
snakes. The term "rattlesnake" may have come from the fact that
the cottonmouth vibrates its tail vigorously when nervous as do
many other snakes, or it may have been confused with rattlesnakes.
Because of the general public's fear of snakes and their reluctance
to learn to discriminate between the poisonous and harmless species,
numerous kinds of snakes seen in or near water have been called
moccasins. The general appearance, pugnacious behavior, and
whitish mouth of water-snakes (Natrix) have earned them a bad
reputation. In fact, a great majority of the "cottonmouths" reported
in many areas are found to be water-snakes.
The cottonmouth is economically important mainly because of
the injurious or fatal effects of its bite and the psychological effect
that its actual or suspected presence has upon many persons. The
species eats a wide variety of prey items and helps to prevent overabundance
of certain kinds of organisms. The venom has been
used in the therapeutic treatment of blood clots owing to its anticoagulant
properties (Didisheim and Lewis, 1956). It also is employed
in the treatment of haemorrhagic conditions and rheumatoid
arthritis, as well as in the production of antivenin (Allen and
Swindell, op cit.:13). None of these uses of venom has become
widely accepted, and its value is questionable.
ACKNOWLEDGMENTS
For guidance in the course of my study, I am especially indebted to Professor
Henry S. Fitch. For suggestions concerning the preparation of the
manuscript, I thank Professor E. Raymond Hall. I am grateful to my wife,
Janis, for her invaluable assistance and for typing the manuscript.
For use of specimens in their care, I thank Professors William E. Duellman,
University of Kansas; Robert L. Packard, formerly of Stephen F. Austin State
College; W. Frank Blair, University of Texas; and William B. Davis and
[441]
Richard J. Baldauf, Texas Agricultural and Mechanical College. Mr. John
E. Werler of the Hermann Park Zoo, Houston, Texas, contributed live individuals;
Mr. Richard S. Funk contributed information on the birth of a brood
of cottonmouths; and Dr. Henry M. Parrish contributed information on the
incidence of snakebite. To numerous other persons at leading museums
throughout the United States for information on the cottonmouths in their
collections, to all who helped with the field work in various ways, and to
others at the University of Kansas for their help and suggestions I am grateful.
SYSTEMATIC RELATIONSHIPS AND DISTRIBUTION
Snakes of the genus Agkistrodon are relatively primitive members of the
Crotalidae, which is one of the most specialized families of snakes. A majority
of the pit-vipers are found in the Americas, but close relatives are found from
extreme southeastern Europe through temperate Asia to Japan (A. halys) and
southeastern Asia including Indonesia (Agkistrodon and Trimeresurus). Familial
characters include: vertical pupil of the eye; facial pit present between
the preoculars and loreal; scales usually keeled; short, rotatable maxilla bearing
a large hollow fang; toothless premaxilla; chiefly hematoxic venom; and undivided
anal plate.
The genus Agkistrodon includes about nine species in the Old World and
three in North and Central America. Some of the primitive characters of the
genus are: head covered with nine enlarged shields or having the internasals
and prefrontals broken up into small scales; subcaudals on proximal part of
tail undivided; fangs relatively short; tail lacking rattles. In one species, A.
rhodostoma, the scales are smooth; and the female is oviparous and guards her
eggs until they hatch. Other species have keeled scales and are ovo-viviparous.
There is little paleontological evidence illustrating evolution of the cottonmouth
or for that matter of crotalids in general. Brattstrom (1954) summarized
the current knowledge of fossil pit-vipers in North America. The few fossils
found of the cottonmouth are from Alacha, Brevard, Citrus, Levy, Pasco, and
Pinellas counties, Florida (Brattstrom, op. cit.:35; Auffenberg, 1963:202). All
are of late Pleistocene Age and well within the present geographic range of the
cottonmouth.
Of crotalid genera only Agkistrodon occurs in both the Old World and the
New World, suggesting that this genus is relatively old. Schmidt (1946:
149-150) mentioned several other closely related groups of animals found in
both eastern Asia and eastern North America, including the reptilian genera: Natrix, Opheodrys, Elaphe, Ophisaurus, Leiolopisma (= Lygosoma), Eumeces, Clemmys, Emmys, and Alligator. Of the groups of animals now confined to
these two regions the most important are the cryptobranchid salamanders, the
genus Alligator, and the spoon-bills (Psephurus in China and Polyodon in the
Mississippi drainage). Fossil evidence for these groups indicates that existing
forms common to eastern Asia and eastern North America are remnants of a
late Cretaceous or early Tertiary Holarctic fauna which was forced southward
as the climate became gradually cooler to the north. "Other clues suggest
that both Agkistrodon and Trimeresurus (Bothrops) moved from Asia to
America, one of these presumably giving rise to the rattlesnakes." (Darlington,
1957:228).
[442]
The named, American kinds of Agkistrodon currently are arranged as three
species: the copperhead, the cantil and the cottonmouth. The copperhead
(A. contortrix) is divided into four subspecies, all of which are terrestrial. This
species occurs from southern New England to eastern Kansas and along the
Atlantic and Gulf Coastal plains, exclusive of peninsular Florida and the delta
of the Mississippi River in Louisiana. It extends southwest from Kansas through
the Edwards Plateau of west-central Texas. Isolated populations occur in the
Chisos and Davis mountains of Trans-Pecos Texas. The cantil or Mexican
moccasin (A. bilineatus), probably the nearest relative of the cottonmouth
(A. piscivorus), is divisible into two subspecies and occupies a nearly complementary
range from Mexico south to Nicaragua. The cottonmouth occurs
throughout the coastal plains of the southeastern United States, usually at
altitudes of 500 feet or less. Two subspecies are recognized, the eastern A. p.
piscivorus and the western A. p. leucostoma. A revision of the genus is underway
by Professor Howard K. Gloyd.
The basic pattern and various behavioral traits are common to all three
species. The young are more nearly alike in appearance than adults, the
copperhead and the cottonmouth being easily confused. Adults differ in
color, size, body proportions, habitat, and habits. In range and habitat preference
the cottonmouth more closely resembles the southern subspecies of
the copperhead, A. c. contortrix, which is usually found in lowlands, near
swamps and streams, but seldom in water.
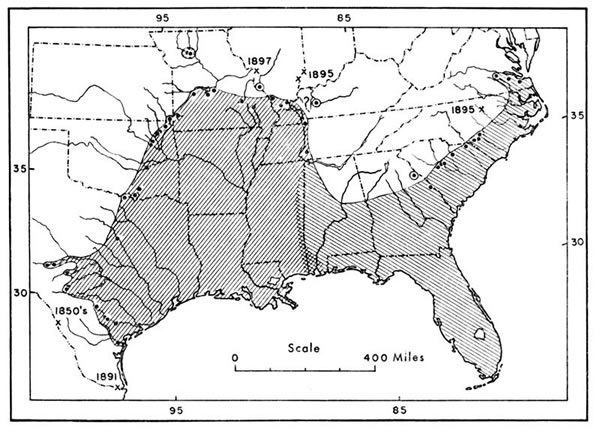 Fig. 1.
Fig. 1. Fig. 1. Geographic range of the cottonmouth, showing marginal and near-marginal
records, based largely upon maps by Gloyd and Conant (1943:165)
and Conant (1958:336) but including additional records. The more important
of these records (from east to west) are discussed in the following paragraphs.
Crosshatching indicates the area of intergradation between the eastern and
western subspecies. Old records, indicated by dates, and their sources are as
follows: 1850's and 1891—U. S. National Museum numbers 4263 and 32753
respectively; 1897—Hurter (1897); and 1895—Stejneger (1895:408).
[443]
The northernmost record for the eastern subspecies is in the Petersburg area,
Prince George County, Virginia (Anon., 1953:24). A sight record (Hickman,
1922:39) near Bristol, West Virginia, probably was based on a water-snake
(Natrix sp.), since the stream in which the snake was seen flows north into the
Ohio River rather than southeast through Virginia. In North Carolina the
most inland record is from the Neuse River, six to eight miles east of Raleigh
(Stejneger, 1895:408). Neill (1947:205) reported a population in the vicinity
of Dry Fork Creek on the boundary line of Wilkes and Oglethorpe counties,
Georgia. Distribution of cottonmouths in Florida is statewide, including the
Keys and other offshore islands.
The ranges of the two subspecies, piscivorus and leucostoma, meet near the
eastern border of Mississippi. A. p. piscivorus has been reported from Tishomingo
County to the Gulf and east of the Loess Bluff area in central Mississippi,
and A. p. leucostoma has been reported from this area westward. A few
specimens from along the Coast indicate intergradation (Cook, 1962:33) between
the two subspecies.
Barbour (1956:33) reported one specimen from Cypress Creek, in the Green
River drainage, Muhlenberg County, Kentucky, and stated that suitable habitat
can be found in several areas east of Kentucky Lake. Hence, cottonmouths
may have entered this area via the Ohio River. Stejneger (loc. cit.)
reported the species in the Wabash River at Mount Carmel, Wabash County,
Illinois, and mentioned a former occurrence at Vincennes, Knox County,
Indiana; but there are no recent records at these localities. Hurter (1897)
reported having seen cottonmouths in Illinois, opposite St. Louis; Smith (1961:265)
believes that this and a population in Monroe County, Illinois, are
isolated relicts, since no specimens have been found within 50 miles to the
south of Monroe County. The specimens reported by Anderson (1941:178;
1945:274) near Chillicothe (three miles southwest and seven miles northwest,
respectively), Livingston County, Missouri, also are thought to represent a relict
population. Hall and Smith (1947:453) reported one specimen from Jasper
County, Missouri, in the Spring River which flows through extreme southeastern
Kansas and into Oklahoma and another in the Neosho River at Chetopa, Kansas.
Both of these specimens were taken after a flood, and no additional
specimens have been taken in this region. Nevertheless, sufficient habitat is
probably available along the Neosho and Verdigris rivers in the southeastern
part of Kansas.
In Texas the cottonmouth has penetrated marginal habitat perhaps farther
than anywhere else in its range. Formerly it was thought to be limited to the
country east of the Balcones Escarpment (Smith and Buechner, 1947:8), but
semiarid areas of the state have been invaded primarily via the Colorado and
Brazos River systems up to altitudes of 2300 feet. Two additional specimens
are said to have been collected along the Rio Grande. Dr. Howard K. Gloyd
(in litt.) stated that the specimen reported from Eagle Pass, Maverick County,
is believed to have been taken in the 1850's; and the one said to have come
from the mouth of the Devil's River is actually marked "near Santa Rosa,
Cameron County, September 30, 1891." No additional specimens have been
taken in that area; and the range now probably extends no farther south than
Corpus Christi, Texas. Brown's (1903:554) knowledge of the extension of
the range of the cottonmouth west of longitude 98° is probably based upon
the records along the Rio Grande reported in the nineteenth century.
Three extensions of the known range in Texas are reported herein. One
specimen was captured by Mr. Harry Green (HWG 346) along the San Saba
River, 8.1 miles west of Menard, Menard County. The other two specimens
(KU 84375 and 84376) were taken by the late Paul Anderson one and one-half
miles north of Pecan Crossing, South Concho River, Tom Green County,
and one mile west of Mertzon, Irion County.
In the hypsithermal period following Pleistocene glaciation, cottonmouths
gradually moved northward occupying areas beyond their present range. The
distributional records since the 1850's and the apparent relict populations now
in existence indicate that the range of this species has since receded.
[444]
DESCRIPTION
Color and Pattern
Color predominantly brown, ranging through pale reddish-brown or dark
reddish-brown, brownish-green, to almost black; 10 to 17 irregular dark brown
bands on paler brown ground color; young paler (some nearly salmon pink),
retaining a vivid pattern throughout first year; pattern of most individuals
nearly obliterated by third year; brilliance and dullness of predominant color
correlated with molting cycle (skin especially bright and shiny immediately
following shedding; tip of tail yellowish in juveniles; posterior part of venter
and tail uniformly black in some adult individuals, especially females; secondary
sexual differences in dorsal coloration, such as found in copperhead by Fitch
(1960:102), not noted.
The eastern subspecies, A. p. piscivorus, has the more brilliant pattern in
which the centers of the dark cross-bands are invaded by the ground color.
The cross-bands are slightly constricted in the mid-line and may or may not be
bilaterally symmetrical. One-half of the cross-band may be displaced anteriorly
or posteriorly to a slight degree or may even be completely absent. From one
to several dark spots may be present within the cross-bands.
The western subspecies, A. p. leucostoma, has a comparatively dull pattern
in which the ground color does not invade the center of the cross-bands. In
many instances the bands are outlined by white scales, as in the Mexican
moccasin (this character is not so prominent in A. p. piscivorus because of
the paler ground color). A large, dark blotch usually occurs at the base of
the cross-band and may completely cross the ventral scales. The characteristic
variations found in piscivorus are also present in leucostoma.
The number of bands is often difficult to count because of the dark color
of some specimens. Gloyd and Conant (1943:168) reported averages of 12.5
(11 to 16) and 12.2 (10 to 16) in males and females, respectively, of leucostoma and ranges of 10 to 17 for males and 10 to 16 for females with averages
of 13 in both sexes of piscivorus. On 20 specimens of leucostoma from Texas
the average number of bands was 12.7 (11 to 15). If the number of bands
differed on the two sides of an animal, the total number of the two sides was
divided by two.
Scutellation
The scutellation of the cottonmouth closely resembles that of the other
species of Agkistrodon. For example, the nine cephalic shields are characteristic
of most species of Agkistrodon, as well as most other primitive crotalids and
viperids, and most colubrids. Most individuals have an additional pair of large
scales behind the parietals.
The numbers of postoculars, supralabials, and infralabials are variable. On
either side the postoculars (three in most specimens) are reduced to two in
some specimens. The supralabials (eight in most specimens) frequently vary
(usually on one side only) from seven to nine. The number of infralabials is
somewhat more variable than the number of supralabials, the usual number
being 11, but 10 is also common; 8, 9, and 12 are more rare (Table 1). In
102 snakes in which these characters were examined, four different combinations
of supralabials and seven combinations of infralabials were found. Both
characters together yielded 16 combinations, considering only the actual number
of scales and not taking into account the side of the head on which they
occurred (Table 2). The combinations found in a brood of seven young from
Houston, Texas, are shown in Table 3 to illustrate the variability of this character.
Gloyd and Conant (1943:168) found a variation of 6 to 11 (8) and 7
to 9 (8) supralabials and 8 to 13 (11) and 8 to 12 (10.4) infralabials in
samples of 301 leucostoma and 119 piscivorus, respectively (numbers in
parentheses represent average). Also of interest is the variability of the scales
themselves. In one instance a scale was found that had not completely divided.
In another specimen the last supralabial and last infralabial were one scale
that completely lined the angle of the jaw. Instances of one scale almost [445] crowding out another were common. In still other instances one or two
supralabials were divided horizontally into two scales. Individual variation
rather than geographical variation occurs in these characters.
TABLE 1.—Frequency of Occurrence of Various Numbers of Supralabial and
Infralabial Scales in 102 Cottonmouths.
| Number of scales | Specimens having number on both sides | Specimens having number on one side | Total | Percentage |
| Supralabials | ||||
| 7 | 11 | 24 | 35 | 25.2 |
| 8 | 64 | 27 | 91 | 65.5 |
| 9 | 0 | 3 | 3 | 2.2 |
| Infralabials | ||||
| 8 | 0 | 2 | 2 | 1.5 |
| 9 | 3 | 10 | 13 | 9.6 |
| 10 | 12 | 32 | 44 | 32.4 |
| 11 | 53 | 22 | 75 | 55.1 |
| 12 | 0 | 2 | 2 | 1.5 |
TABLE 2.—Numbers of Supralabials and Infralabials of 102 Cottonmouths.
| Number of individuals | Number of supralabials | Number of infralabials |
| 37 | 8 | 11 |
| 15 | 8 | 10-11 |
| 12 | 7-8 | 11 |
| 6 | 7-8 | 10-11 |
| 5 | 8 | 10 |
| 5 | 8 | 9-10 |
| 4 | 7 | 11 |
| 3 | 7 | 9-10 |
| 3 | 7-8 | 10 |
| 2 | 7 | 9 |
| 2 | 7 | 10 |
| 2 | 8 | 10-12 |
| 2 | 8-9 | 10 |
| 2 | 7-8 | 8-9 |
| 1 | 7-8 | 9 |
| 1 | 8-9 | 10-11 |
The dorsal scales of cottonmouths are strongly keeled except that those of
the two lower scale-rows on each side are weakly keeled. Also they are
slightly larger than the others. Two apical pits are present on each dorsal
scale. The shape of the scales and number of scale rows vary depending upon
the position on the body. Scales on the neck are considerably smaller than
those elsewhere on the body and are arranged in two or three more rows than
those at mid-body. The skin in the region of the throat, neck, and fore-body is [446] especially elastic and allows the swallowing of large prey. Posteriorly from the
mid-body the scales decrease in size and become more angular, those on the
tail tending to be rhomboidal and wider than long. In the region of the anus
the number of scale rows diminishes rapidly, leaving only 12 to 14 rows at the
base of the tail and only three rows immediately ahead of the tail tip. The
tail ends in a spine composed of two scales: one scale covers the bottom, lower
parts of the sides, and tip of the spine; and a shorter dorsal scale covers the
top and upper parts of the sides of the basal two-thirds of the spine. The
spine of embryos and young cottonmouths is blunt, but is pointed in most
adults.
TABLE 3.—Variation in Numbers of Supralabials and Infralabials in a Brood
of Seven Cottonmouths.
| Number of individuals | Number of supralabials | Number of infralabials |
| 1 | 7 | 9 |
| 1 | 7 | 9-10 |
| 2 | 7-8 | 8-9 |
| 1 | 7-8 | 9 |
| 1 | 8 | 9-10 |
| 1 | 8-9 | 10 |
TABLE 4.—Analysis of Number of Scale Rows at Three Parts of the Body
in 81 Cottonmouths.
| Number of scales per row | Neck | Mid-body | Anterior to anus | |||
| Number of individuals | Percentage | Number of individuals | Percentage | Number of individuals | Percentage | |
| 29 | 1 | 1.2 | ||||
| 28 | 3 | 3.7 | ||||
| 27 | 52 | 64.2 | ||||
| 26 | 16 | 18.0 | 2 | 2.5 | ||
| 25 | 8 | 9.9 | 67 | 82.7 | ||
| 24 | 1 | 1.2 | 4 | 4.9 | ||
| 23 | 8 | 9.9 | 4 | 4.9 | ||
| 22 | 4 | 4.9 | ||||
| 21 | 68 | 84.0 | ||||
| 20 | 5 | 6.2 | ||||
The number of scale rows on the neck, at mid-body, and just anterior
to the anus is relatively constant at 27-25-21, respectively; but some individual
variation is evident (Table 4). Since the rows are diagonally arranged, it is
necessary in counting scales to proceed either anteriorly or posteriorly across
the back; or the row may be counted in either direction up to the center of the
back and then reversed on the other side of the snake. In order to count the
scale rows in a position where no scale reduction or addition was occurring
and to avoid as much error as possible, I counted from anterior to center and
back on the neck, in any direction at mid-body, and from posterior to center
and back near the anus. Because females generally are the larger in circumference
posteriorly, they could have more scale rows than males just anterior [447] to the anus. The few snakes having more than 21 scale rows in the posterior
region offer no conclusive evidence as to tendencies, but in both instances in
which this occurred the females outnumbered the males three to one. An
odd, rather than an even, number of scale rows occurs on most of the length
of the snakes examined, because there is a mid-dorsal row and scale rows tend
to be lost on both sides at about the same level. An example of scale reduction
of one snake was as follows:
| 6 + 7 (13) | 6 + 7 (96) | |||
| 27 ————— | 25 ————— | 24 ————— | 23 ————— | 22 ————— |
| 5 + 6 (13) | 5 + 6 (90) | 7 + 8 (111) | 7 + 8 (114) | |
| 6 + 7 (122) | + 7, -5 (125) | |||
| 23 ————— | 22 ————— | 23 ————— | 21 ————— | 22 ————— |
| -6 (118) | + 6 (119) | 6 + 7 (121) | + 6 (123) | |
| -6 (126) | ||||
| 22 ————— | 21 (130). | |||
This scale reduction follows the method proposed by Dowling (1951b: 133)
in which the numbers on the mid-line represent the number of scale rows,
upper figures refer to the right side of the snake, and figures in parentheses indicate
the number of the ventral scale (counted from the anterior end of the
series), thus marking the position of the addition or reduction. Addition of
a row is shown by a plus sign and the number of the row, whereas reductions
are shown by a minus sign and the number of the row that is lost or by a
plus sign between the number of two rows that join. According to Dowling,
variation in number of dorsal scales characterizes the few genera and species
of snakes in which it has been studied. The time and difficulty involved in
ascertaining the number of scales explain why it has not been widely used in
classification.
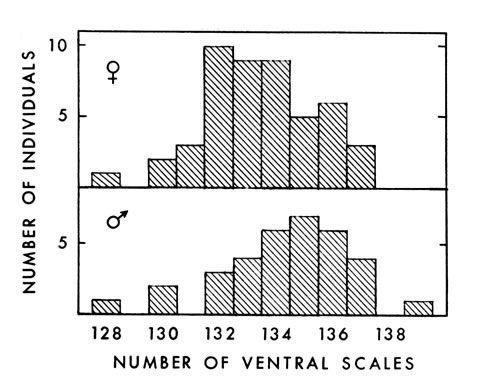 Fig. 2. Number of ventral scales in 48 female and 34
Fig. 2. Number of ventral scales in 48 female and 34male A. p. leucostoma.
Ventral scales on 34 males averaged 134.4 (128 to 139), and on 48 females
133.5 (128 to 137) (Fig. 2.). Barbour (1956:34) found an average of 135.3
ventral scales on 64 males and 44 females, and Gloyd and Conant (loc. cit.)
found an average of 134 for both males and females. The average for the
eastern cottonmouth obtained by Gloyd and Conant, however, was 137 ventrals
in both sexes. Some of my counts were made before I knew of the standard
system of counting ventrals proposed by Dowling (1951a:97-99), in which [448] the first ventral plate is defined as the most anterior one bordered on both
sides by the first row of dorsals. Therefore, some inconsistencies may exist in
my counts. Where differences occur, Dowling's method probably will indicate
the presence of an additional scale, since it appears to begin farther anteriorly
on the average, than I began counting.
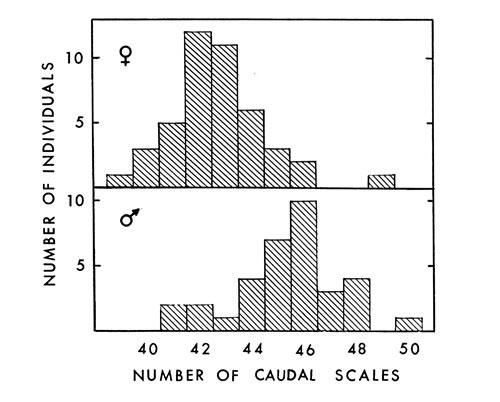 Fig. 3. Number of caudal scales in 44 female and 34
Fig. 3. Number of caudal scales in 44 female and 34male A. p. leucostoma.
TABLE 5.—Caudal Scale Combinations in 95 Cottonmouths. U = Undivided;
D = Divided.
Number | Number of scales | ||||||||||||||||
| D | U | D | U | D | U | D | U | D | U | D | U | D | U | D | U | D | |
| 25 | 13-35 | 10-32 | |||||||||||||||
| 11 | 1-2 | 12-33 | 14-28 | ||||||||||||||
| 20 | 16-39 | 1-9 | 1-3 | 3-24 | |||||||||||||
| 20 | 1-4 | 3-37 | 1-21 | 1-5 | 1-29 | ||||||||||||
| 4 | 14-30 | 1-8 | 1-7 | 1-8 | 1-4 | 2-10 | |||||||||||
| 3 | 1 | 18-23 | 1-2 | 1-2 | 6-11 | 1-3 | 6-9 | ||||||||||
| 4 | 1-17 | 1 | 1-3 | 1-8 | 1-4 | 1-3 | 1-4 | 13-22 | |||||||||
| 2 | 1-2 | 4-16 | 1 | 1-4 | 2 | 1 | 1-4 | 1 | 18-21 | ||||||||
| 1 | 20 | 1 | 1 | 1 | 1 | 6 | 1 | 3 | 1 | 11 | |||||||
| 1 | 10 | 2 | 3 | 2 | 10 | 1 | 2 | 2 | 1 | 4 | 4 | ||||||
| 1 | 20 | 1 | 1 | 2 | 1 | 1 | 4 | 4 | 2 | 4 | 1 | 3 | |||||
| 1 | 1 | 13 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 4 | 2 | 4 | 13 | ||||
| 1 | 17 | 1 | 1 | 2 | 1 | 1 | 6 | 2 | 1 | 2 | 3 | 2 | 7 | ||||
| 1 | 9 | 1 | 1 | 8 | 1 | 3 | 1 | 1 | 3 | 1 | 1 | 2 | 1 | 1 | 1 | 6 | |
Analysis of caudal scales revealed sexual dimorphism. In the six specimens
from Tennessee, Blanchard (1922:16) found the same thing. Caudals averaged
45.4 (41 to 50) on 34 males and 42.6 (39 to 49) on 44 females (Fig. 3).
Barbour (loc. cit.) found an average of 45.7 (30 to 54) caudals in males and
43 (17 to 56) in females. Caudal scale counts by Gloyd and Conant (loc.
cit.) averaged 44 (38 to 49) in males and 42 (37 to 48) in females of leucostoma;
in piscivorus they averaged 48 (42 to 53) in males and 44 (41 to 49)
in females. Another seldom-mentioned, unusual characteristic of the caudal [449] scales of copperheads and cottonmouths is that some are single (usually those
at the base of the tail) and others divided (Table 5). To my knowledge, all
other species have either single or divided scales the entire length of the tail.
See Klauber (1941:73) and Fox (1948:252) concerning correlation of few
scales with warm environment.
Dentition
Cottonmouths, like other pit-vipers, have their teeth reduced in number
and have enlarged, highly specialized fangs. Small teeth occur on the palatine
and the pterygoid in the upper jaw and on the dentary in the lower jaw. The
dentary bone bears 17 curved teeth that decrease in size posteriorly. The
palatine bears five small, strongly curved teeth, and the pterygoid bears 16 to
18 strongly curved teeth decreasing in size posteriorly. The numbers of teeth
mentioned above in each instance refer to the number of sockets rather than
the actual number of teeth, because teeth are frequently shed, leaving some
of the sockets empty at any one time.
The maxillary bone has two sockets side by side which bear the poison
fangs, usually one at a time. During the period shortly before a fang is to be
shed, however, its replacement becomes attached in the alternate socket; and
both fangs may be functional for a short time. The old fang then becomes
weakened at its base, eventually breaks off, and is swallowed. At any one
time four or five replacement fangs in various stages of development are found
in the gum behind the functional fang. These replacement fangs, which are
arranged in alternate rows, gradually enlarge as they move forward in their
development and, in juveniles, are generally slightly longer than the fangs that
they replace.
In 1963 I examined the fangs of 14 cottonmouths at four- to seven-day intervals
for a period of six weeks. The fang-shedding cycle was found to be
highly irregular, with a double condition (on one or both sides) occurring one-third
of the time. Approximately the same proportion of double fangs was
found in preserved individuals. A replacement period of at least five days
was observed in one snake. One-half the cycle (from replacement on one side
to replacement on the other) varied from five to twenty days, indicating that
the cycles for each fang are independent of one another. Bogert (1943:324)
found that young rattlesnakes are born with functional fangs in the two inner
sockets. Nonsynchronous use of the sockets on opposite sides of the head in
rattlesnakes is a later development which results from accidents or other conditions
leading to a longer retention of the fang on one side than on the other
(Klauber, 1956:723). I found a double set of fangs in cottonmouths only
twice in the six-week period. A complete cycle was recorded in ten instances
in a period of 19 to 23 days and in two instances in 32 days. One cottonmouth
was examined periodically over a 34-day period by Allen and Swindell
(1948:12), but a complete fang-shedding cycle was not observed. Fitch
(1960:110) reported a 33-day cycle in copperheads; Klauber (1956:726)
estimated the normal active life of each fang of an adult rattlesnake to be
from six to ten weeks, but he made no observations to confirm his estimation.
Fangs measured from the tip of the notch of the basal lumen to the end of
the fang vary from about 1.3 per cent of the snout-vent length in juveniles to
about 1.0 per cent in large adults (Table 6). The fangs are longer than those
of copperheads (Fitch, 1960:111). Klauber's (1956:736) figures on fang-lengths
in all species of rattlesnakes are percentages of total length rather than
of the snout-vent length. The fangs of various species of rattlesnakes range
from nearly the same proportionate length as those of cottonmouths to some
much longer.
From patterns of bites of venomous snakes, Pope and Perkins (1944:333-335)
attempted to correlate number, size, and patterns of tooth marks with
size and generic identity of the snake responsible for the bite. Distance between
fangs is relatively constant for snakes of a particular size (Table 6) regardless
of genus, but the fangs of a cottonmouth are directed outward to
variable degrees, and puncture wounds could easily resemble those of a much
larger snake (Table 7). Also there is no direct relationship between size of [450] snake and toxicity or amount of venom injected. Consequently information of
this kind is of little or no value from a medical standpoint.
TABLE 6.—Correlation of Relative Fang-length and Distance Between Fangs
at Base with Snout-vent Length of Cottonmouths.
| Snout-vent length (millimeters) | Number | Average | Number
| Average ratio of distance between fangs to snout-vent length (percent) |
| 200-299 | 3 | 1.33 | 3 | 2.57 |
| 300-399 | 7 | 1.30 | 5 | 2.48 |
| 400-499 | 13 | 1.21 | 9 | 2.21 |
| 500-599 | 12 | 1.22 | 8 | 2.19 |
| 600-699 | 7 | 1.17 | 1 | 2.10 |
| 700-799 | 5 | 1.07 | 4 | 1.65 |
| 800-899 | 1 | 1.00 | 1 | 2.00 |
TABLE 7.—Contrast in Measurements Between the Base of the Fangs and
Between Fang Punctures of Nine Cottonmouths (in millimeters).
| Distance between base of fangs | Distance between fang punctures | Snout-vent length |
| 7.7 | 13.0 | 400 |
| 8.7 | 14.0 | 575 |
| 10.0 | 22.5 | 526 |
| 11.0 | 18.0-19.0 | 590 |
| 12.0 | 18.0 | 793 |
| 13.0 | 17.0, 20.0 | 558, 612 |
| 15.5 | 23.5 | 800 |
| 16.0 | 24.0 | 800 |
HABITAT AND LIMITING FACTORS
Although usually associated with swamps and lowlands along river bottoms,
the cottonmouth lives in a variety of habitats ranging from salt marshes to cool,
clear streams and from sea level to an altitude of 2300 feet. Shaded, moist
areas either in or beside shallow waters are preferred, but cottonmouths occasionally
wander as far as a mile from water.
In the pine-oak forests of Nacogdoches County in eastern Texas cottonmouths
and copperheads are probably the most abundant species of snakes.
Specimens have been collected near Nacogdoches in ponds, swamps, clear and
fast-running streams with rock bottoms, and sluggish muddy streams. On the
Stephen F. Austin Experimental Forest numerous cottonmouths live in a
swamp until around mid-July, when it becomes dry. A small stream west of [451] the swamp seems to be used as a migration route to and from the swamp.
Slightly more than a mile downstream cottonmouths are common in a bottomland
area. The ground is always moist and no undergrowth occurs; a few
small clear springs produce shallow trickles that run into a swamp. Cottonmouths
can often be found here, lying in or beside the small trickles.
I have seen cottonmouths in various types of aquatic habitats in Brazoria
County. In most places in this area, cottonmouths are found in association
with one or more species of water-snakes (including Natrix cyclopion, N.
erythrogaster, N. rhombifera, and N. confluens), which greatly outnumber the
cottonmouth. Interspecific competition may be reduced somewhat by cottonmouths
sometimes feeding on water-snakes.
The numerous statements in the literature concerning the habitat of the
cottonmouth can be summarized most easily by the following short quotations:
Agkistrodon piscivorus piscivorus—"Marshes and lakes; ponds and streams
with wooded shores; low country near water; roadside ponds; drainage ditches;
coastal 'banks'; keys; some Gulf coast islands; mangrove swamps." (Wright
and Wright, 1957:919.)Agkistrodon piscivorus leucostoma—"Cypress, gum, river swamps; alluvial
swamps wooded or not wooded; water courses of the south such as rivers,
bayous, backwaters of small branches; hill streams in the north; ...
marshy places in prairies ... rice fields, bottomland pools; margins of
above habitats, pools, shallow lakes, swampy places, temporary flood lands.
... In, under, or on fallen timber, in holes in banks, rocky bluffs, crayfish
burrows. In short it is very aquatic." (Wright and Wright, op. cit.:923.)
Geographically cottonmouths differ somewhat in their ecological requirements,
but are basically much alike in most respects. The areas of greatest
abundance are those having 40 inches or more of annual rainfall. The northern
edge of the range has a mean temperature of approximately 38° F. in January
in Virginia and 30° F. in Missouri, although the lowest temperature reached
in these areas is more important as a limiting factor. The annual rainfall in
both Virginia and Missouri amounts to approximately 40 inches. Moisture,
as well as temperature, may play an important role in the northward distribution
of the species. The eastern cottonmouth seems to be less tolerant of low
temperatures than the western subspecies. Mean January temperatures equal
to those along the northern limits of the western cottonmouth's distribution
are reached in the vicinity of Connecticut, which is north of the geographic
range of the eastern subspecies.
The depths to which cottonmouths penetrate into their dens may have a
limiting influence upon the geographic range, especially in the northern extremes.
Bailey (1948:215) discussed the possibility that populations of snakes
may be significantly depressed because of winter kill of individuals that "hibernate"
at shallow depths. He speculated also that the short growing season
does not allow enough time for the essentials of existence to be carried out,
and the prolonged period of inactivity overtaxes the energy reserve of the
species.
Available food does not seem to be of much importance as a limiting factor,
for the cottonmouth is remarkably indiscriminate in its choice of prey, feeding
upon almost any vertebrate animal that happens to come within reach. Competition
for food, however, may play an important role.
[452]
REPRODUCTION
Courtship and Mating
A review of available literature indicates no records of courtship of the
cottonmouth other than statements that breeding occurs in early spring. In
a close relative, the copperhead (see Fitch, 1960:159-160), mating occurs
almost any time in the season of activity but is mainly concentrated in the
few weeks after spring emergence, at about the time when females are ovulating.
Klauber (1956:692) concluded that along the southern border of the
United States rattlesnakes normally mate in spring soon after coming out of
their winter retreats; but farther north where broods are produced biennially,
the mating times may be more widely dispersed, and summer and fall matings
may even predominate.
The only record of copulation in the cottonmouth was reported by Allen
and Swindell (1948:11), who observed a pair copulating for three hours on
October 19, 1946, at the Ross Allen Reptile Institute. Davis (1936:267-268)
stated that courtship in cottonmouths is violent and prolonged but did not
note any nervous, jerky motions or nudging of the female along her back and
sides as had been observed in other genera of snakes. Carr (1936:90) saw
a male cottonmouth seize a female in his mouth and hold her, but no courtship
followed.
Reproductive Cycles
Many persons have assumed that gestation periods in snakes are the intervals
between mating and parturition, and that mating and ovulation occur at approximately
the same time. However, retention of spermatozoa and delayed
fertilization indicate that copulation is not a stimulus for ovulation.
A biennial reproductive cycle was found for the copperhead in Kansas
(Fitch, 1960:162), the prairie rattler in Wyoming (Rahn, 1942:239) and in
South Dakota (Klauber, 1956:688), the great basin rattler in Utah (Glissmeyer,
1951:24), and the western diamondback rattler in northwestern Texas (Tinkle,
1962:309). Klauber's (1956:687) belief that the reproductive cycle of rattlesnakes
varies with climate, being biennial in the north and annual in the
south, is supported by similar climatic variation in the reproductive cycle of
the European viper which was discussed by Volsøe (1944:18, 149).
If data for a large number of females were arranged as are those in Table 8,
they might reveal whether the breeding cycle is annual or biennial. The figures
presented in Table 8 are misleading if viewed separately because of the small
number of individuals included in some of the size classes.
The smallest reproductive female found measured 455 millimeters in snout-vent
length. Conant (1933:43) reported that a female raised in captivity
gave birth to two young at an age of two years and ten months. The size
classes represented by gravid females found by Barbour (1956:38) in Kentucky
indicate that breeding occurs at least by the third year.
The ovaries of female cottonmouths examined revealed ova in various stages
of development. In individuals less than 300 millimeters in snout-vent length
the ovaries are almost completely undeveloped; in immature individuals from
300 to 450 millimeters in length the follicles are from one to two millimeters
in length; in post-post females follicles vary in size, the largest being about
seven millimeters. Reproductive females also contain follicles of various sizes. [453] One or two sets are less than three millimeters in length, and large ova that
soon are to be ovulated are present. Ovarian ova found in April ranged in
length from 23 to 35 millimeters. No embryonic development was observed
in most individuals until June or later.
TABLE 8.—Percentage of Gravid Females of A. p. leucostoma in
50 Millimeter Size Classes.
| Snout-vent length | Number of gravid females | Total number in size class | Percentage gravid |
| 450-499 | 3 | 14 | 21.4 |
| 500-549 | 7 | 17 | 41.2 |
| 550-599 | 8 | 17 | 47.1 |
| 600-649 | 5 | 7 | 71.4 |
| 650-699 | 2 | 9 | 22.2 |
| 700-749 | 2 | 3 | 66.7 |
| 750-799 | 1 | 1 | 100.0 |
| 850-899 | 1 | 1 | 100.0 |
| Totals | 29 | 69 | 42.0 |
Increase in length of testes appears to be correlated with length of the
individual rather than cyclic reproductive periods (Fig. 4).
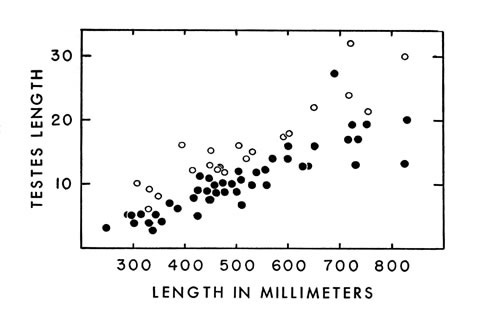 Fig. 4. Length of testes in cottonmouths of various sizes
Fig. 4. Length of testes in cottonmouths of various sizes ( ·—left; °—right ). The right testis is always longer than
the left.
The reproductive cycle in cottonmouths resembles that illustrated by Rahn
(op. cit.:237), in which the ovarian follicles of post-partum females begin to
enlarge in late summer and autumn, with ovulation occurring the following
spring. By means of retaining sperm successive broods possibly are produced
after only one mating. In captivity, at least, some females may not follow
this biennial cycle; Stanley Roth (M.S.), biology teacher in high school at
Lawrence, Kansas, had a female of A. p. piscivorus, from Florida, that produced
broods of 14 and 12 young in two consecutive years.
[454]
Embryonic Development
After ova are fertilized a three and one-half to four-month period of development
begins which varies somewhat depending on the temperature. In
almost every instance the ova in the right uterus outnumber those in the left.
Embryos usually assume the serpentine form in the latter part of June and
are coiled in a counterclockwise spiral with the head on the outside of the
coil. At this time the head is relatively large and birdlike in appearance with
conspicuous protruding eyes. Sex is easily noted because the hemipenes of
males are everted. By late July scales are well developed and the embryo is
more snakelike in appearance, but pigmentation is still absent. By mid-August
the color and pattern are well developed, the egg tooth is present, the snake
shows a considerable increase in size over that of the previous month, and
much of the yolk has been consumed. Some females that contain well developed
embryos also contain eggs that fail to develop. Sizes of ova vary
irrespective of size of female and stage of embryonic development. Lengths
of ova ranged from 22 to 51 millimeters in May to 35 to 49 millimeters in
July and August. A two-yolked egg was found in one female.
Birth of Young
Accounts in the literature of 15 litters of cottonmouths fix the time of
birth as August and September. Conant (1933:43) reported the birth of a
litter in mid-July by a female that had been raised in captivity, and one female
that I had kept in captivity for two months gave birth to a litter between
October 19 and October 25. The conditions of captivity undoubtedly affected
the time of birth in both instances.
Wharton (1960:125-126) reported the birth and behavior of a brood of
seven cottonmouths in Florida. I was given notes of a similar nature by
Richard S. Funk of Junction City, Kansas, on a brood of five cottonmouths.
The mother of the brood was caught in June, 1962, in Tarrant County, Texas,
by Richard E. Smith, and was 705 millimeters in snout-vent length. The first
young was found dead in an extended position a few inches from the fetal
membranes at 11:05 p.m. on August 22. The second young was born at
11:07 p.m. The intervals between the successive births were three, seven,
and four minutes; and time until the sac was ruptured in each instance was
six, five, eight, and 11 minutes. The time interval between the rupture of the
sac and emergence of each individual was 41, 92, 154, and 34 minutes. The
mother's actions in giving birth to the last four young were essentially as
described by Wharton (loc. cit.), except that the intervals between successive
births did not increase. Within one minute after rupturing the sac and while
its head was protruding, each of the four living young opened its mouth
widely from three to seven times, then took its first breath. Breaths for the
first three hours were steady at three or four per minute but then decreased
to two or three per minute. Pulse rate for the four averaged 38 per minute
while at rest but increased to 44 per minute after voluntarily crawling.
Number of Young per Litter
Records of from one to 16 young per litter have been reported (Ditmars,
1945:330; Clark, 1949:259), but the average is probably between six or seven.
Most accounts in the literature present information on number of ova or embryos [455] per female rather than the number of young. Size and age of the
mother (Table 9) influence the number of ova produced. Allen and Swindell
(1948:11) recorded three to 12 embryos in 31 cottonmouths varying in total
length from 26 to 44 inches. An average of 6.5 embryos per female was
found.
TABLE 9.—Number of Ova Produced by Fecund Cottonmouths.
| Snout-vent length in millimeters | Number in sample | Number of ova, average and extremes |
| 450-549 | 10 | 4.1 (2 to 7) |
| 550-649 | 11 | 4.9 (1 to 8) |
| 650-749 | 4 | 6.3 (4 to 8) |
| 750-849 | 1 | 5 |
| 850-949 | 1 | 14 |
Mortality at birth has been recorded for almost every litter born in captivity
(see Allen and Swindell, loc. cit.; Conant, 1933:43; Wharton, 1960:125).
A female that I kept in captivity gave birth to seven young. Three never
ruptured their sacs, and another died soon after leaving the sac. The effects
of captivity on females may result in higher rates of deformity and mortality
in young than is common in nature. Klauber (1956:699-700) estimated that
the defects brought about by conditions of captivity on rattlesnakes eliminate
about three young per litter.
Population Composition
No investigator has yet analyzed the composition of a population of cottonmouths
according to age, sex and snout-vent length. Barbour (1956:35) did
sort 167 snakes into size classes, but did not determine sex ratio, size at sexual
maturity, reproductive cycles, or snout-vent length. He recorded total lengths
from which snout-vent lengths cannot be computed because of differential
growth rates and different bodily proportions of the two sexes. I judge from
my findings that he included immature individuals in his three smallest size
classes (45.5 per cent of the population). I found at least 32.5 per cent immature
individuals (Fig. 5) in my material, but it was not a natural population.
The sex ratios of several small collections from natural populations varied,
and no conclusions could be drawn. Females comprised 53 per cent of the
specimens included in Fig. 5 and in a group of 48 embryos which represented
eight broods. That percentage may not be the percentage in a natural population
but is used in making assumptions because I lack better information.
Reproductive Potential
If data in Fig. 5 are representative of a natural population and if 61 per
cent of the females are sexually mature, the reproductive potential can be
estimated as follows: assuming a cohort of 1000 cottonmouths contains 530
females, 61 per cent of the females (323 individuals) probably are adults.
If 42 per cent of these females produce 6.5 young per female in any season
(Tables 8 and 9), 136 females will produce 884 young. But if 50 per cent [456] of the adult females are reproductive (as would be assumed if reproduction
is biennial), 1050 young will be produced. Actually the number of young
required per year to sustain a population is unknown, because mortality rates
at any age are unknown.
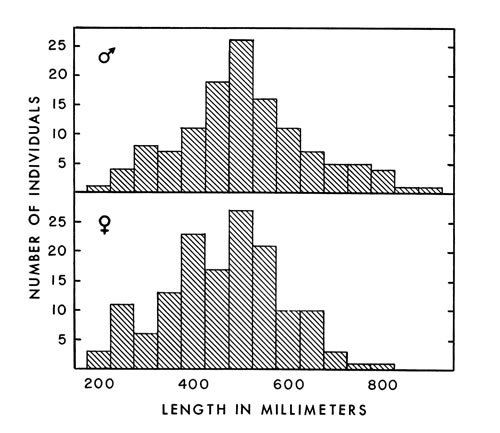 Fig. 5. Composition of a group of cottonmouths examined in this
Fig. 5. Composition of a group of cottonmouths examined in thisstudy. Individuals less than 450 millimeters in snout-vent length
are considered as immature. Specimens from 200 to 249 millimeters
in length are included in the 200-millimeter class, etc.
GROWTH AND DEVELOPMENT
Size at Birth and Early Growth
Size at birth depends on the health of the mother. According to Fitch
(1960:182), many litters of copperheads born in captivity are stunted. Seven
young cottonmouths (two males and five females) born in captivity were each
185 millimeters in snout-vent length and 40 millimeters in tail length. Weights
of the three living young were 10.0, 10.1, and 11.1 grams. Another litter of
five young measured by Richard S. Funk were larger, and differences in the
proportions of the tail length and snout-vent length suggest the sexual dimorphism
found in larger individuals. However, sex of these young snakes
was not recorded. Snout-vent length and tail length in millimeters were 232,
41; 243, 47; 229, 40; 240, 48; and 225, 40 in the order of their birth. These
snakes are considerably smaller than the nine young of A. p. piscivorus reported
by Wharton (1960:127) that averaged 338 millimeters total length and 28.7
grams. The yolk of one young piscivorus was 11.7 per cent of the total weight. [457] Yolk is used up in about two weeks if its rate of utilization resembles that of
the copperhead as reported by Gloyd (1934:600).
Early rates of growth of three living young are shown in Table 10. On
the 56th day after birth, each was fed one minnow less than two inches long.
Between the 80th and 120th days three additional small minnows were fed
to each snake. Young cottonmouths increase nearly 50 millimeters in length
by the first spring if they inhabit warm areas and feed in autumn or winter.
Variation in size of newborn cottonmouths may be less in nature than in
captivity. Average size at birth can be determined accurately by the size of
young captured in early spring, at least in northern parts of the range where
winter feeding and growth do not occur at all or are negligible. Total lengths
of 19 juveniles thought by Barbour (1956:38) to be seven to eight months old
do not differ markedly from lengths of the five newly-born young measured by
Funk.
TABLE 10.—Rate of Growth of Three Young Cottonmouths.
| Age in days | Snout-vent length / tail length—weight in grams | ||
| Female No. 1 | Female No. 2 | Male | |
| 2 | 185/40—11.1 | 185/40—10.1 | 185/40—10.0 |
| 7 | 192/40— | 190/40— | 189/40— |
| 22 | 195/40—10.3 | 200/41.5—10.6 | 197/40— |
| 80 | 204/40—11.7 | 203/42—10.4 | 218/48—14.3 |
| 88 | .... | 204/44— | .... |
| 143 | 215/40.5—13.3 | .... | 225/48—15.1 |
The Umbilical Scar
The umbilical cord is broken at birth and the navel closes within a few
days; but the scar, involving from two to four ventral scales, remains throughout
life. Position of the scar was found by Edgren (1951:1) to be sexually
dimorphic in the eastern hog-nose snake (Heterodon platyrhinos), but nothing
has been published on this matter concerning the cottonmouth. Consequently,
I counted the scales of several individuals from the anal plate, and there was
no marked difference in the position of the scar in males and females; it varied
in position from the 10th to the 18th scale. When counted from the anterior
end, the scar ranged from ventral number 115 to 122 (average, 119) in 28
females and from number 117 to 126 (average, 121) in 14 males. The difference
between male and female cottonmouths is not nearly so great as in Heterodon.
Later Growth and Bodily Proportions
The only records of growth increments in a natural population of cottonmouths
are those in Table 11. The period of growth is mostly the period of
activity, and differences are expected between northern and southern populations.
As size increases, determination of growth rate becomes more difficult
because age classes overlap in size. Growth of any individual depends not
only on climate and food but also on disease and parasitism and the innate [458] size potential. Stabler (1951:91) showed weight and length relationships in
two cottonmouths for a period of six and one-half years.
TABLE 11.—Growth Increments in Cottonmouths (Barbour, 1956:38-39).
| Number of individuals | Total length in millimeters | Estimated age in months | Estimated growth from preceding year in millimeters |
| 19 | 260-298 | 7-8 | 25 |
| 11 | 312-337 | 19-20 | 45 |
| 40 | 355-485 | 31-32 | 95± |
| 83 | 500-1000 | 43-44+ | ? |
My study failed to reveal any secondary sexual difference in growth rate
and maximum size. Of the 306 cottonmouths measured by me, 16 males and
five females exceeded 700 millimeters in snout-vent length. Two males were
more than 850 millimeters long. One cottonmouth lived in captivity for 18
years and 11 months (Perkins, 1955:262). The maximum total lengths were
reported by Conant (1958:186-187) to be 74 inches (1876 mm.) in A. p.
piscivorus and 54 inches (1370 mm.) in A. p. leucostoma.
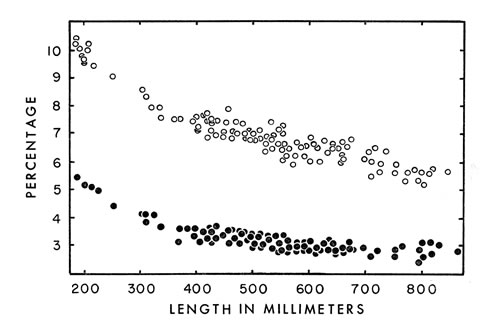 Fig. 6. Head length ( ° ) and head width ( · ) expressed as a percentage
Fig. 6. Head length ( ° ) and head width ( · ) expressed as a percentageof snout-vent length of living and preserved cottonmouths. Head length
was measured from the tip of the snout to the posterior end of the mandible.
Head width was measured across the supraocular scales, since accuracy was
greater than if measured at the posterior edge of the jaw. No sexual dimorphism
or geographical variation occurs in these characters.
Proportions of various parts of the body vary considerably depending on
age, size and, in some instances, sex. Heads are proportionately larger in
young than in adults (Fig. 6), as is true of vertebrates in general. This
larger head has survival value for the cottonmouth in permitting more venom [459] to be produced and in permitting it to be injected deeper than would be the
case if the proportions were the same as in adults. Relative to the remainder
of the snake the head is considerably larger than in the copperhead (Fitch,
1960:108) and slightly larger than in the rattlesnake, Crotalus ruber (Klauber,
1956:152).
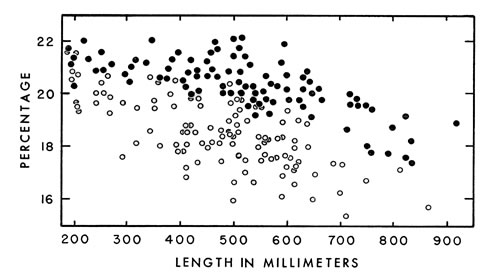 Fig. 7. Tail length expressed as a percentage of snout-vent length of living and preserved cottonmouths ( ·—males; °—females ).
Fig. 7. Tail length expressed as a percentage of snout-vent length of living and preserved cottonmouths ( ·—males; °—females ). In general, tails are relatively longer in males than in females of the same
size (Fig. 7), except that there is little or no difference at birth. Growth of
the tail in males proceeds at a more rapid rate. In certain individuals sex
cannot be recognized from length of the tail relative to snout-vent length because
overlapping occurs, especially in medium-sized individuals. Similar
changes of proportions with increase in age occur in copperheads (Fitch, 1960:106)
and rattlesnakes (Klauber, 1956:158-159), but the tail of the cottonmouth
is relatively much longer.
SHEDDING
The Shedding Operation
Shedding of the skin is necessary to provide for growth and wear in snakes.
The milkiness or bluing of the eyes, which causes partial blindness, marks the
initial stage of shedding and is caused by a discharge of the exuvial glands that
loosens the old stratum corneum from the layer below. In four to seven days
the opaqueness disappears, and the snake sheds after an additional three to six
days (Table 12). Young snakes first shed within a few days after birth and
generally shed more frequently than adults, but the interval is variable. The
eyes of three young cottonmouths observed by Wharton (1960:126) became
milky on the fourth day but cleared on the seventh day, and the skin was
shed on the eighth day. The eyes of three young kept by me became milky
two to three days after birth, cleared on the seventh to tenth days, and the
skin was shed on the thirteenth day. Possibly the relatively long interval in [460] this instance resulted from low relative humidity in the room where the
snakes were kept. According to Fitch (1960:134), litters of young copperheads
usually shed within three to ten days after birth; but under unusually
dry conditions shedding did not occur for several weeks.
TABLE 12.—Duration of Preparatory Period (in days) to Shedding in 11
Cottonmouths.
| Duration of cloudiness of eyes | Time between clearing and shedding | Time from beginning of cloudiness until shedding |
| 5 | 6 | 11 |
| 7 | 3 | 10 |
| - | - | 6 |
| - | - | 6 |
| 5 | 3 | 8 |
| 4 | 6 | 10 |
| 7 | 3 | 10 |
| 5 | 6 | 11 |
| 5 | 3 | 8 |
| 7 | - | - |
| 7 | 3 | 10 |
| X 5.4 | X 3.8 | X 9.0 |
Cottonmouths as well as other snakes usually do not feed until after the
skin is shed and are generally quiescent during the period preceding shedding,
except that immediately before shedding they become active and rub their
snouts on some rough object and may yawn several times seemingly in an
attempt to loosen the skin along the edges of the lips. After the skin is
loosened from the head, more rubbing against rough surfaces and writhing
serves to pull the old skin off, turning it inside out. Once the old skin has
passed over the thick mid-body, the snake often crawls forward using rectilinear
locomotion until the skin is completely shed. It normally comes off in
one piece; but, if the snake is unhealthy or has not had sufficient food or
water, the skin may come off in patches. Frequently one or both of the
lens coverings are not shed immediately and impair the sight. Bathing or
swimming ordinarily causes dried skin to peel off; and, because of the cottonmouth's
aquatic habits, its chances of shedding successfully are much greater
than those of less aquatic snakes. Cottonmouths that have recently shed
have bright and glossy patterns, in contrast to the dull and dark appearance
of those that are preparing to shed.
Frequency of Shedding
Most of our knowledge concerning the frequency of shedding is based
upon observations of captives. It is known that the intervals between exuviations
are largely dependent upon the amount of food taken and the rate of
growth. Unless laboratory conditions closely resemble those in the field,
shedding frequencies in captives probably differ much from those of free-living
snakes.
Only two of my captives shed twice. The intervals between exuviations [461] in the two snakes were eight and five months, lasting from August to April
and from December to May, respectively. Ten other snakes shed once in
the period from January through July. Stabler (1951:91) presented data
concerning shedding of two cottonmouths kept 12 and 14 years in captivity.
One shed 25 times in 12 years and the other shed 37 times in 14 years, giving
an average of 2.1 and 2.6 per year, respectively. Neither of the snakes
shed from December through March, but the period of shedding corresponded
to the period of greatest activity and growth. In Florida, cottonmouths shed
four to six times a year, according to rate of growth (Allen and Swindell,
1948:7).
FOOD HABITS
Methods of Obtaining Prey
Food is obtained by a variety of methods depending on the type of food,
age of the cottonmouth, and possibly other factors. Some captives lie in
ambush and others crawl slowly in active search. At the first cue of possible
prey, either by sight, scent, or differential temperature detection by the pit,
the snake appears to become alert and flicks its tongue out at fairly rapid
intervals.
By means of the facial or loreal pit found in all crotalids, the snake is able
to detect objects having temperatures different from that of the surroundings
of the objects. In detecting prey the tongue acts to sharpen the sense of
"smell" by conveying particles to Jacobson's organs in the roof of the mouth.
On many occasions cottonmouths appeared to rely solely on sight; they
passed within a few inches of prey, apparently unaware of its presence until
it moved. When pools of water begin to dry up toward the end of summer,
cottonmouths often congregate and feed on dying fish. In these instances
the fish are usually taken as they come to the surface. In laboratory observations
moccasins seize live fish and some moccasins carry the fish until they
have received lethal doses of venom; afterward the fish are swallowed. But
grasping and manipulation of the prey occurs without the fangs' being employed,
especially in the case of dead fish. On one occasion a cottonmouth
was observed to grasp the edge of a glass dish that had contained fish and
apparently retained the odor. On another occasion I placed several fish in a
bowl, rubbed a stick on the fish, and then touched each snake lightly on the
nose with the stick. The snakes crawled directly to the bowl and began
feeding. At other times these same snakes crawled around the cage in an
apparent attempt to locate the food but paid little attention to fish held in
front of them. If the catching of prey under natural conditions were as
uncoordinated as it sometimes is in captivity, the snakes probably would not
be able to survive.
Wharton (1960:127-129) described tail-luring in one individual of a 76-day-old
brood of cottonmouths. The snake lay loosely coiled with the tail
held about six centimeters from the ground; a constant waving motion passed
posteriorly through the terminal inch of the tail. These movements ceased
at 7:20 p.m. but were resumed at 7:40 a.m. the following day. All observations
were under artificial light. The "caudal lure" as a means of obtaining
prey has been described in other species and related genera by Neill (1960:194)
and Ditmars (1915:424).
Various authors have suggested that the method of capture differs according
to the kind of prey. Allen and Swindell (1948:5) stated that cottonmouths
retain their hold after striking fish or frogs but will release a mouse after
delivering a bite and are timid in striking at larger rodents. Neill (1947:203)
noted that a cottonmouth always waited several minutes after biting a large
rat before approaching its prey. This same type of behavior has been reported
for copperheads (Fitch, 1960:194) and rattlesnakes (Klauber, 1956:618). [462] Cottonmouths observed by me retained a strong hold on fish, frogs, and
sometimes mice, but almost always released large mice and baby chicks,
which were not eaten until after death.
Different behavior according to type of prey is correlated with ability of
prey to retaliate, although some animals may not be released because they
could easily escape. For instance, a frog could hop far enough to escape in
a matter of seconds if released. A 73-millimeter Rana pipiens that I observed
was bitten twice within one and a quarter hours and died 45 minutes after
the last bite. Its movement was uncoordinated by the time of the second bite,
but it could have escaped had the frog not been confined. Although it is
doubtful that normal, healthy fish are frequently captured by cottonmouths,
Allen (1932:17) reported that a cottonmouth was seen pushing a small,
dead pike about on the surface of a stream. A wound on the belly of the
fish indicated that it had been bitten. A 17-gram creek chub (Semotilus)
and a 13.7-gram bass (Micropterus) were injected by me with one-fourth
cubic centimeter of fresh venom near the base of the tail in order to determine
whether the fish could escape after being bitten and released. The creek
chub flipped onto its back after a minute and 45 seconds and gill movements
stopped in eight minutes and 35 seconds; the bass flipped over after 50 seconds
and died in two minutes and 10 seconds. The venom immediately affected
both fish, and it is unlikely that either could have swum more than a few feet.
After its prey has been killed, a cottonmouth examines the body from end
to end by touching it with the tongue. Then the animal is grasped in the
mouth without the use of the fangs and is slowly manipulated until one end
(usually the head) is held in the mouth. The lengthy process of swallowing
then takes place, the fangs and lower jaws alternately pushing the prey down
the throat.
Food and Food Preferences
The cottonmouth seems to be an opportunistic omni-carnivore, because it
eats almost any type of flesh that is available, including carrion. It feeds
primarily upon vertebrates found in or near water; but invertebrates and
eggs have also been found in the diet. The only potential prey items that
seem not to be normally eaten are bufonid toads and tadpoles. I have
occasionally offered tadpoles and frogs to cottonmouths, but only the frogs
were accepted. But, Stanley Roth kept a cottonmouth in captivity that ate
both toads and tadpoles. If tadpoles are commonly eaten, their probable
rapid digestion would make identification almost impossible.
Following is a list of known foods of the cottonmouth:
Captivity: "... rattlesnake.... The same moccasin also killed
and ate a smaller snake of its own species...." (Conant, 1934:382.)Florida: "3 heron feathers, bird bone, Eumeces inexpectatus, 3 fish all under
one inch in length, 1 heron egg shell" (Carr, 1936:89). According to Allen
and Swindell (1948:5), "the food included other moccasins, prairie rattlesnakes,
king-snakes, black snakes, water snakes, garter snakes, ribbon snakes,
and horn snakes ... most of the species of frogs, baby alligators, mice,
rats, guinea pigs, young rabbits, birds, bats, squirrels, and lizards ...
a mud turtle ... a case of a four footer eating ten to twelve chicken
eggs. The most common food appears to be fish and frogs. Catfish are
included on this list...." Yerger (1953:115) mentions "an adult yellow
bullhead, Ameiurus natalis ... 306 mm. in standard length [from a 63-inch
cottonmouth]."
[463]
Georgia: "... full grown Rana catesbeiana, several foot-long pickerel
... dead fish if placed in a pan of water.... Natrix sipedon
fasciata and Masticophis flagellum ... rats.... Toads and large Eumeces laticeps were always ignored." (Neill, 1947:203.) "Natrix, Heterodon, Kinosternon, Rana, Hyla cinerea, Microhyla, Microtine [Pitymys pinetorum]."
(Hamilton and Pollack, 1955:3.)Mississippi: "... Hyla gratiosa.... In captivity specimens have
eaten frogs, mice, birds, dead fish, pigmy rattlers and copperheads. Toads
... were refused" (Allen, 1932:17). One moccasin "disgorged a smaller
decapitated moccasin ... killed the day before by boys" (Smith and
List, 1955:123).Tennessee: "Beetles in one stomach; lizard (Eumeces) in another stomach;
small snake (Natrix) in one intestine, and hair in another intestine. One
stomach contained numerous bits of wood, up to four inches in length...."
(Goodman, 1958:149.)Kentucky: "Siren intermedia was the most abundant food item in both
volume and occurrence. Frogs of the genus Rana ranked second. Together,
these two items comprised almost 2/3 of the food of the snakes. The other
food items were distributed among the fishes, reptiles, and other amphibians
[one Rana tadpole included]." (Based on 42 samples—Barbour, 1956:37.)Illinois: (Based on 84 samples—Klimstra, 1959:5.)
Food Item Per cent Frequency
of OccurrencePer cent
VolumePisces 39.3 31.9 Amphibia 36.9 26.0 Reptilia 25.0 18.2 Mammalia 30.9 17.9 Gastropoda 17.8 1.0 Miscellaneous 25.0 5.0 (Algae, Arachnida, Aves, Insecta) Louisiana: Penn (1943:59) mentions that a "female had just eaten two
young cottonmouths...." Clark (1949:259) mentions "100 specimens—34
fish; 25 Rana pipiens; 16 Rana clamitans; 7 Acris; 4 Natrix sipedon confluens;
8 birds; 5 squirrels ... catfish thirteen and one-half inches in length
... small-mouth black bass [eleven inches]."Oklahoma: Force (1930:37) remarks that the moccasin "eats bullfrogs
... but refuses leopard frogs." Trowbridge (1937:299) writes: "several
sun perch.... Another had eaten six catfish six to ten inches long
... a water snake (Natrix s. transversa) about 18 inches long ...
frogs, mostly Rana sphenocephala." Carpenter (1958:115) mentions "a
juvenile woodthrush.... Seven last instar cicadas ... a young
cottontail." According to Laughlin (1959:84), one moccasin "contained the
following items: 18 contour feathers of a duck, probably a teal; one juvenile
cooter turtle, Pseudemys floridana; and a large mass of odd-looking unidentifiable
material. The other cottonmouth contained one juvenile pond turtle, Pseudemys scripta...."Texas: "... several ... feeding on frogs.... One ...
found DOR was found to contain a large catfish." (Guidry, 1953:54.)
Of 246 cottonmouths that I examined for food items, only 46 contained
prey in their digestive tracts. Almost all of the snakes examined were
museum specimens that had been collected at many places over a period
of about 40 years. It was not known how long each had been kept alive
before being preserved. Therefore it was impossible to determine what
proportion of any population of cottonmouths could be expected to contain
food. The food items were not analyzed numerically because the scales and
hair, by means of which many food items in the intestine were identified,
yielded no clue as to the number of individuals actually present unless several [464] distinct kinds were found. Each occurrence of scales or hair was thus recorded
as a single individual, although some such occurrences may have represented
more than one animal. The contents of some stomachs were so well digested
that it was difficult to determine the number of items present. As a rule
only one food item was present in a digestive tract, but a few tracts contained
several items of the same or different species. Three frogs (Acris crepitans)
were in one snake and three hylas (Hyla versicolor) in another. Still another
individual captured beside a drying pond contained six individuals of Lepomis each about three inches long and two pikes (Esox) about six inches long.
TABLE 13.—Analysis of Food Items of 46 Cottonmouths Collected in
Arkansas, Louisiana, and Texas (1922-1962).
| Food Items | Number of samples in which item occurred | Percent frequency of occurrence | Estimated weight in grams | Estimated percentage by bulk | |
| Fish | (7) | 13.2 | 18.4 | ||
| Esox sp. | 1 | 20 | |||
| Lepomis sp. | 2 | 15 | |||
| Unidentified | 4 | ||||
| Amphibians | (12) | 23.0 | 20.4 | ||
| Scaphiopus hurteri | 1 | 13 | |||
| Acris crepitans | 2 | 4 | |||
| Hyla cinerea | 2 | 12 | |||
| Hyla versicolor | 1 | 12 | |||
| Rana catesbeiana | 1 | 20 | |||
| Rana pipiens | 3 | 15 | |||
| Unidentified | 2 | ||||
| Reptiles | (15) | 28.4 | 29.9 | ||
| Pseudemys scripta | 2 | 15 | |||
| Anolis carolinensis | 1 | 6 | |||
| Eumeces fasciatus | 1 | 7 | |||
| Lygosoma laterale | 2 | 5 | |||
| Natrix sp. | 1 | 10 | |||
| Natrix erythrogaster | 2 | 10 | |||
| Agkistrodon piscivorus | 2 | 20 | |||
| Crotalus sp. | 1 | 30 | |||
| Unidentified snakes | 3 | ||||
| Birds | (4) | 7.6 | 18.6 | ||
| Anhinga anhinga (juv.) | 1 | 60 | |||
| Egret (head and neck) | 1 | 20 | |||
| Passeriformes | 2 | 20 | |||
| Mammals | (6) | 11.3 | 12.7 | ||
| Blarina brevicauda | 1 | 12 | |||
| Cricetinae | 5 | 18 | |||
| Unidentified | (9) | 17.0 | |||
The "unidentified" category (Table 13) refers to jellylike masses in the
stomach or material in the intestine in which no scales, feathers, hair, or bones [465] could be found. Most of the unidentifiable matter could be assumed to
consist of remains of amphibians, since they leave no hard parts. If this
assumption is correct, amphibians comprise about 40 per cent of the diet.
Since intestinal contents were included, a volumetric analysis was not feasible.
Therefore, the weight of each type of food item was estimated and the
percentage by bulk calculated from it (Table 13).
Pieces of dead leaves and small sticks constituted most of the plant material
found and presumably were ingested secondarily because they adhered to the
moist skin of the prey, especially to fish and amphibians. However, some
plant materials probably are eaten because they have acquired the odor of
the prey. One cottonmouth contained a Hyla cinerea, several leaves, and five
sticks from 37 to 95 millimeters long and from 12 to 14 millimeters in diameter.
Most reports in the literature state that gravid females do not feed, but
four gravid females examined by me containing large, well-developed embryos
also contained evidences of having recently fed. Two of them had scales of
snakes in the stomach or intestine, one contained a six-inch Lepomis, and the
other had hair in the intestine and the head and neck of an adult egret in the
stomach.
MORTALITY FACTORS
Natural Enemies and Predators
Published records of other animals preying on cottonmouths or killing them
are few. Reptiles more often than other classes of vertebrates prey on the
cottonmouth. McIlhenny (1935:44) reported on the scarcity of snakes in
areas where alligators were present. Predation on cottonmouths by indigo
snakes (Drymarchon corais) was reported by Conant (1958:153) and Lee
(1964:32). Allen and Swindell (1948:6) obtained a photograph of a king-snake
(Lampropeltis getulus) killing a cottonmouth but thought that moccasins
are not eaten by L. getulus. However, one occasion reported herein shows that
cottonmouths are eaten by king-snakes; and Clark (1949:252) reported finding
13 cottonmouths, along with other prey, in the stomach contents of 301 king-snakes
(L. g. holbrooki) from northwestern Louisiana. Cannibalism is also
common among cottonmouths. Klauber (1956:1058;1079) cited predation
on cottonmouths by a blue heron (Ardea herodias) and a largemouth bass
(Micropterus salmoides). Man is probably the greatest enemy of the cottonmouth.
Intentional killing, capturing, road kills, and alteration of the environment
destroy large numbers.
Parasites and Diseases
Allen and Swindell (1948:12) listed several diseases and parasites of snakes
and stated that "some moccasins captured in the woods are so poor and weak
from parasitic infection that they can barely crawl." The only kind of ectoparasite
found on captive cottonmouths in the course of my study was a snake
mite, Ophionyssus natricus. An infestation of that mite was thought to be
partly responsible for the death of one captive moccasin. Other moccasins
spent increasing amounts of time in their water dish after they became
infected with mites. Under natural conditions frequent swimming probably
keeps cottonmouths nearly free of mites.
Endoparasites found included lung flukes, stomach nematodes, and tapeworms.
Lung flukes (Ochetosoma sp.) were found in 16 of 20 captive cottonmouths. [466] Snails and frogs serve as intermediate hosts for various stages in
the life cycle of these flukes. The high percentage of cottonmouths infested
with flukes is indicative of the use of frogs as a major source of food. Less
than ten flukes were usually observed in the snakes' mouths but occasionally
more were seen. One snake was observed thrashing about in its cage for
nearly an hour, after which time it died. Upon examination of the mouth,
32 flukes were found, most of which were located in the Jacobson's organs.
Whether or not flukes caused the death is not known. Nematodes (Kalicephalus sp.) were found in the stomach of each of several preserved specimens;
most of these snakes had no food in their digestive tracts. In a high percentage
of the moccasins, tapeworms (Ophiotaenia sp.) were in the duodenum,
in many instances so tightly packed as seemingly to prevent passage of food.
The importance of fish in the diet is reflected by the high percentage of
snakes containing tapeworms. An unidentified cyst (?) about an inch long
and containing two hooks on one end was found attached to the outer wall
of the stomach of a cottonmouth. Yamaguti (1958) listed all the kinds
of helminths known from cottonmouths.
Miscellaneous Causes of Death
Munro (1949:71-72) reported on the lethal effect of 10 per cent DDT
powder on two young cottonmouths which were dusted with it to kill mites.
Herald (1949:117) reported an equal effect caused by spraying a five per cent
DDT solution in a room with several snakes. All but three large cottonmouths,
which were under shelter at the time of spraying, were killed.
One individual that refused to eat was dissected soon after death, and a
short piece of a branch on which two large thorns were located at 90° angles
was found blocking the intestine at the posterior end of the stomach.
An unexpected and probably unusual circumstance caused the death of two
captives. After cleaning a cage containing five cottonmouths and placing
several mice in the cage for food, I noticed two of the snakes lying stretched
out, partially on one side, and almost unable to move. At first I thought they
had been bitten by other snakes which were in pursuit of the mice. The two
died after two days. When a similar incident occurred in another cage, I
removed the "bitten" snake and it fully recovered after 11 days. When
the same symptoms were observed in a garter snake in another cage, I
realized that in each instance the cage had been cleaned and fresh cedar
chips placed in it immediately prior to observation of these symptoms. Fine
cedar dust on the chips had evidently poisoned the snakes.
BEHAVIOR
Annual and Diel Cycles of Activity
In the days following emergence in spring, cottonmouths often endure uncomfortable
and even dangerous temperatures in order to obtain food and
mates. They are more sluggish at this time and more vulnerable to predation
than later in the season when temperatures are optimal. Fitch (1956:463)
found that copperheads in northeastern Kansas begin their annual cycle of
activity in the latter part of April, when the daily maximum temperature is
about 22° C. and the minimum is about 4° C., and become dormant in late [467] October or early November, at which time the daily maximum temperature is
about 15° and the minimum is about 0°. Indications are that in the northern
part of its range the annual activity cycle of the cottonmouth resembles that
of the copperhead in northeastern Kansas. Klimstra (1959:2) captured cottonmouths
from April to October in southern Illinois. Barbour (1956:36) collected
large numbers of them in early April in Kentucky and stated that they
migrate from swamps to wooded hillsides in late August and early September.
Spring migrations begin after a few consecutive warm days in March. In
northern Oklahoma cottonmouths have been found along the Verdigris River
as early as March, suggesting that a few winter in crayfish holes and mammal
burrows. The majority of individuals found in this area were at denning sites
along cliffs above the river and emerged later than those near the river (Dundee
and Burger, 1948:1-2). In Virginia cottonmouths have been seen as early as
March 5 (Martin and Wood, 1955:237) and as late as December 4. They
have been observed in migration from the swamps of the barrier beach to the
mainland in late October and early November in southeastern Virginia (Wood,
1954a:159). According to Neill (1947:204), the cottonmouth tolerates lower
temperatures than do most snakes in Georgia and is one of the last to go into
hibernation. Allen and Swindell (1948:4) stated that cottonmouths usually
bask during the mornings of the cooler months in Florida, but they mentioned
nothing of denning such as occurs farther north. Although winter aggregations
occur in the northern parts of the range, I have never seen such aggregations
in the South. However, in one instance related to me by a reliable
observer, seven cottonmouths were found together on a creek bank near the
Gulf Coast in early spring.
During late summer and early autumn, fat is deposited in lobes in the lower
abdomen in preparation for the period of winter quiescence. Gravid females
usually do not feed so frequently or so much as other snakes, because they
tend to become inactive as the ova develop. Whether or not females feed
heavily after parturition and previous to denning is not known. Peaks of
activity in autumn may be caused by final attempts to feed before denning
and by the appearance of large numbers of newborn young. The young
usually have from one to two months in which to feed before the advent of
cold weather. According to Barbour's (op. cit.:38) findings, the young probably
feed before hibernation because they grow substantially in winter. For
those that do not feed, the rate of survival is perhaps much lower.
In preparation for winter, cottonmouths migrate inland, usually to dry
forested hillsides where they den, commonly among rocks at the tops of bluffs,
along with several other species of snakes. In such aggregations there is no
hostility and each individual may derive benefit from contact with others by
which favorable conditions of temperature and humidity are maintained.
Neill (1947:204) has found many specimens in winter by tearing bark from
rotting pine stumps on hillsides overlooking lakes or streams. On cold days
they evidently retreat below the surface, while on warm days they lie just
below the bark or emerge and bask. Neill believes that the use of stumps by
cottonmouths is an innate pattern of behavior, because of the large number
of young-of-the-year found in such surroundings. Cottonmouths were observed
in winter also under logs and stumps by Allen (1932:17). I have
twice observed cottonmouths crawling into crayfish burrows along the Gulf
Coast of Texas, and suppose they are used as denning sites to some extent.
[468]
The diel cycle of activity of cottonmouths is of necessity closely related
to the seasonal cycle. Since optimal temperatures determine activity, the
diel cycle varies greatly from time to time. It has been well established
that cottonmouths, like most other crotalids and many snakes of other families,
prefer nocturnal to diurnal activity, even though the temperature may be
less favorable at night. This preference is correlated with increased nocturnal
activity of frogs and reptiles that constitute the principal food supply.
During spring and autumn, activity is more restricted to the day and long
periods of basking occur. However, as hot weather approaches, basking
occurs mainly in the morning and evening and activity becomes primarily
nocturnal. But, in well shaded, moist forests, cottonmouths feed actively in
the daytime.
Availability of food also has an important influence upon activity. Allen and
Swindell (op. cit.:5) stated that moccasins congregate around drying ponds
and feed on dying fish until the moccasins can hold no more. They then
usually stay nearby as long as food remains. In an area of the Stephen F.
Austin Experimental Forest near Nacogdoches, Texas, many cottonmouths
journey daily to and from a swamp and a dry field, evidently to feed on
rodents inhabiting the area. Ten individuals captured along a snake-proof
fence that was built 30 yards from the swamp were found lying coiled along
the fence after 4:30 p.m., at which time the area was shaded. On another
occasion, I captured a large cottonmouth that was feeding upon dying fish
in a drying pool about 10:30 a.m. on August 19, 1962.
Because of the aquatic habits of the cottonmouth, relative humidity probably
has little influence on the snake's activity. However, cottonmouths are
more restricted to the vicinity of water in dry weather than during rains or
muggy weather when many of their natural prey species also move about
more freely. Increased activity on cloudy days may result from protection
from long exposure to sunshine. Torrential rains and floods, such as those
following hurricanes along the Gulf and Atlantic coasts of the southeastern
United States, bring out quantities of snakes of all species. Rattlesnakes and
cottonmouths in particular are killed by the thousands at these times because
they seek shelter in human habitations. However, these are unusual circumstances
and do not reflect voluntary activity as a result of preferences.
Thermal reactions of reptiles were classified by Cowles and Bogert (1944)
into several categories. For each species there is a basking and normal
activity range limited by the voluntary minimum and voluntary maximum at
which the animal seeks shelter. Beyond this normal range are the critical
thermal minimum and critical thermal maximum (C. T. M.) at which effective
locomotion is prevented. The lethal minimum and maximum are those
temperatures at which short exposure produces irreparable damage, and death
inevitably results. These classifications are modified somewhat by seasonal
or laboratory acclimation or by the physiological state of the animal. The
C. T. M. of five cottonmouths was determined by placing each individual
in an enclosed area and heating it with an infrared lamp. Cloacal temperatures
were taken with a Schultheis quick-recording thermometer as soon as
the snake could no longer right itself when placed on its back. All temperatures
were in degrees Celcius. The C. T. M. averaged 39.2° (38.0° to 40.0°). A
temperature of 38.0° was lethal to one individual. These cottonmouths had [469] been in captivity for nine months. The behavior of the snakes during heating
resembled those instances described by Klauber (1956:382-387) for rattlesnakes.
As the body temperature of the snakes rose past the optimum, each
individual became disturbed and tried to escape from the enclosure. The
snakes soon became frantic in their efforts to escape. After about five minutes
the mouth was opened and heavy, slow breathing was begun, accompanied by
a loss of coordination and a slowing down of movements. The snakes writhed
spasmodically for a few seconds and then lay still, usually with the mouth
open. Recovery was begun by rolling on the belly and flicking the tongue,
followed by movements of the head and then the body. Cottonmouths are
rarely exposed to dangerously high temperatures owing to their semi-aquatic
habits, but there are probably occasions when individuals reach the C. T. M.
for the species.
Basking
Since activity, digestion, and gestation depend upon adequate internal
temperatures, there must be a process by which these temperatures are attained
and for an appropriate time maintained. Basking is important in this respect.
The cottonmouths prefer to lie in a coiled position and, during basking, can
usually be found beside bodies of water or on branches of dead trees overhanging
the water. They are good climbers and have a prehensile tail that is
frequently employed in descending from small branches. Since cottonmouths
are semi-aquatic and are often exposed to temperatures that are lower than
those of the air, they either must bask more often than terrestrial snakes
or tolerate lower temperatures. Length of the period of basking is determined
not only by amounts of insolation and temperature but also by the size of the
snake. A smaller snake can reach its optimum temperature more rapidly
because of a higher surface-to-volume ratio. Another factor that may play
a minor role in the rate of temperature change is the color of the snake. The
wide variation in color of cottonmouths probably affects rates of heat increase
and loss due to direct radiation. Slight hormonal control of melanophores
described in snakes by Neill and Allen (1955) also may exert some influence
on the length of time spent basking. No rates of temperature increase or
decrease are available for cottonmouths.
Coiling
While inactive the cottonmouth spends most of its time lying in a coiled
position with the tail outermost, with the body usually wound into about one
and one-half cycles, and the head and neck in a reversed direction forming a
U- or S-shaped loop. From this position the snake is able to make a short
strike or a hasty getaway if necessary. In my opinion this position is used
primarily for basking or resting and only secondarily for feeding. Most
individuals appear to pursue their prey actively, not lying in ambush for the
approaching prey to the extent that most other crotalids do.
Many of the cottonmouths that I kept in captivity were observed in a coiled
position for periods up to three or four days. Under natural conditions, however,
they are more active. Young cottonmouths are inclined to remain in a
coiled position for longer periods than older individuals.
[470]
Locomotion
Four distinct types of locomotion have been described in snakes: horizontal
undulatory, rectilinear, sidewinding, and concertina (Klauber, 1956:
331-350). Most snakes are capable of employing two or more of these types
of progression, at least to a certain degree; but horizontal undulatory locomotion
is the most common method used by the majority of snakes, including
the cottonmouth. In this method the snake's body is thrown into lateral
undulations that conform with irregularities in the substrate. Pressure is
exerted on the outside and posterior surface of each curve, thus forcing the
body forward.
Rectilinear locomotion is more useful to large, thick-bodied snakes which
use this method of progression, chiefly when they are prowling and unhurried.
This method depends upon the movement of alternate sections of the venter
forward and drawing the body over the ventral scales resting on the substratum
by means of muscular action. This mode of locomotion was most
frequently observed in captive cottonmouths when they were crawling along
the edge of their cages, especially when they were first introduced to the cages
and toward the end of the shedding process. The other two types of locomotion,
sidewinding and concertina, have not, to my knowledge, been observed
in the cottonmouth.
Both the cottonmouth and the cantil have definite affinities for water and
are as likely to be found in water as out of it. Copperheads and rattlesnakes,
although not aquatic, are good swimmers. When swimming, a motion resembling
horizontal undulatory progression is used.
Disposition
The number of different opinions expressed in the literature concerning the
cottonmouth's disposition is not at all surprising. As with any species there is
a wide range of individual temperament, which is affected by many factors.
The cottonmouth is considered by some writers to be docile while others consider
it to be highly dangerous. Allen and Swindell (1948:7) described the
variability in temperament, even among individuals. They wrote: "On rare
occasions, moccasins are found which will attack. A perfectly docile snake
will turn and bite viciously without any apparent reason." They also recounted
a case in which a cottonmouth was kept as a pet for six years, being
allowed the freedom of the house. Smith and List (1955:123) found them
"... surprisingly docile in the gulf region [Mississippi], displaying none
of the pugnacity of more northern cottonmouths." Smith (1956:310) stated:
"Unlike the copperhead, cottonmouths are pugnacious; their powerful jaws,
long fangs, vicious disposition and potent venom make them a very dangerous
animal."
My own observations are in general agreement with the statements of Allen
and Swindell (loc. cit.). In my encounters with cottonmouths, I have never
found any aggressive individuals except for three juveniles that were born in
captivity. In their first three days in the laboratory these juveniles were observed
to strike repeatedly whenever anyone entered the room. After this short
period of aggressiveness, however, they slowly became more docile. The disposition
shown by the newborn young is clearly an innate behavioral pattern
that undoubtedly has a direct relationship to survival. The majority of cottonmouths [471] that I have approached in the field have moved swiftly to seek refuge
in nearby water; a few have remained motionless as I approached, and one
showed the typical threat display. Upon capture and handling, they react
similarly to other pit-vipers by opening and closing the mouth and erecting
the fangs in an attempt to bite. They often bite through the lower jaw and
eject venom at this time as well as when the mouth is open. Of more than a
dozen individuals kept in captivity, four were particularly difficult to handle
whereas another was extremely docile. It was almost never found in aggregations
with the other snakes and did not struggle or attempt to bite when
handled. The majority remained unpredictable in disposition, usually appearing
docile and lazy but capable of extremely rapid movements when disturbed.
Defense and Escape
The typical threatening posture of rattlesnakes is all but lacking in the
cottonmouth, which relies primarily on concealing coloration or nearness to
water for escape. When approached, it usually plunges into nearby water or
remains motionless with the head held up at a 45° angle and the mouth
opened widely exposing the white interior. The tail is sometimes vibrated
rapidly and musk is expelled. This threat display is unique to cottonmouths;
although it does not attract as much attention as the display of rattlesnakes,
it is probably an effective warning to most intruders at close range.
Neill (1947:205) reported one case in which a cottonmouth used the "body
blow" defense, described for Crotalus by Cowles (1938:13), when approached
by a king-snake, Lampropeltis getulus. In this unusual posture the anterior
and posterior portions of the body are held against the ground and the middle
one-fourth to one-third of the body is lifted up and used in striking the
intruder. This same defense posture also was observed in rattlesnakes when
presented with the odor of the spotted skunk, Spilogale phenax. However, the
"king-snake defense posture" is probably not a well-established behavioral
pattern in the cottonmouth, for it sometimes feeds upon king-snakes. I observed
the killing and devouring of a cottonmouth by a speckled king-snake, L. g. holbrooki; the only attempts to escape were by rapid crawling and biting.
Cottonmouths often squirt musk as a defensive action. The tail is switched
back and forth, and musk is emitted from glands on each side of the base of
the tail. The fine jets of musk are sprayed upward at about 45° angles for a
distance of nearly five feet. How often this defense mechanism is used against
other animals is not known, but the musky odor can frequently be detected in
areas where cottonmouths are common. The odor is repulsive and, if concentrated,
can cause nausea in some individuals. To me, the scent is indistinguishable
from that of the copperhead.
"Head Bobbing"
"Head bobbing" in snakes has been described frequently in the literature,
and many interpretations have been advanced to explain its occurrence. One
of the earlier accounts was that of Corrington (1929:72) describing behavior
of the corn snake, Elaphe guttata. Characteristic bobbing occurred when the
snake was cornered, and seemingly the purpose was to warn or frighten foes.
Neill (1949:114-115) mentioned the jerking or bobbing of the head in several
species of snakes including the cottonmouth, and remarked that "it is apparently [472] connected with courtship and with the recognition of individuals."
According to Munro (1950:88), "head bobbing" appears to be a sign of
annoyance in some instances but is usually concerned with reproduction and
individual recognition. Richmond (1952:38) thought that many types of
head movements among not only reptiles but also birds and some mammals
are a result of poor vision and serve "to delimit and orient an object that for
lack of motion is otherwise invisible." Head movements undoubtedly occur
in animals to facilitate accommodation, but it is obvious from Richmond's
conclusions that he has never observed "head bobbing" in snakes. The term
itself is grossly misleading and should be discarded. Mansueti (1946:98)
correctly described the movements as spastic contractions of the body. I have
observed numerous instances of these movements in cottonmouths, copperheads,
and rat snakes (Elaphe obsoleta); and in no case has the movement
resembled a head bob as is described in lizards and other animals. The movement
appears to be a result of a nervous or sexually excited state and consists
of highly spastic contractions confined to the anterior part of the snake most
of the time but affecting the entire body on some occasions.
I found the response to be most common among cottonmouths in confinement
when food was introduced to a cage containing several individuals
(increasing the tendency to strike at a moving object) and when an individual
was placed back in the cage after being handled. At these times the snakes
that were inactive began to jerk for a few seconds. When the snake is in
this seemingly nervous state, the same response is elicited by another snake
crawling over it. At other times the movement of one individual causes no
such response. The jerking movements appear to be released by the recognition
of a nervous state in another individual and may serve to protect the
jerking individual from aggressive advances of the former.
Where courtship is involved, the jerking motions are made in conjunction
with writhing of the male and do not result from the same type of releaser
described above.
Combat Dance
The so-called combat dance between male snakes has long been known,
but its significance is still poorly understood. It was for many years believed
to be courtship behavior until the participants were examined and found to
be males. Carr and Carr (1942:1-6) described one such instance in two
cottonmouths as courtship. In their observations, as well as those of others,
copulation was never observed following the "dance" but was assumed to be
the ultimate goal. After the discovery that only males participated, it was
suggested that combat involved competition for mates, but the "dance" has
been observed at times other than the breeding season (Ramsey, 1948:228).
Shaw (1948:137-145) discussed the combat of crotalids in some detail but
drew no conclusions as to the cause of the behavior. Lowe (1948:134) concluded
with little actual evidence that combat among male snakes is solely
for territorial purposes. Shaw (1951:167) stated that combat may occur as
a possible defense against homosexuality. One case of homosexual mating
among cottonmouths was reported (Lederer, 1931:651-653), but the incomplete
description seems to be of normal courtship procedure except that the
"female" tried to avoid the male. Two instances of combat observed between
timber rattlesnakes (C. h. horridus) by Sutherland (1958:23-24) were definitely [473] initiated because of competition for food. More observations are needed before
the significance of the combat can be fully understood.
THE VENOM
Properties of the Venom
The venom and poison apparatus have developed primarily as a means of
causing rapid death in small animals that are the usual prey. As a protective
device against larger enemies, including man, the venom may have some
value; but this was probably unimportant in the evolution of the poison
mechanism. A secondary function of the venom is to begin digestion of tissues
of the prey. Since food is swallowed whole, injection of digestive enzymes
into the body cavity enhances digestion of the prey.
Kellogg (1925:5) described venom as a somewhat viscid fluid of a yellowish
color and composed of 50 to 70 per cent proteins, the chief remaining components
being water and carbohydrates, with occasional admixtures of abraded
epithelial cells or saprophytic microorganisms. Salts, such as chlorides, phosphates
of calcium, magnesium, and ammonium, occur in small quantities. Each
of the components of snake venom has a different effect on the body of the
victim. It was at first believed that there were two types of venoms:
neurotoxic, which acts upon nervous tissue; and haemotoxic, which acts on
blood and other tissues. It has since been found that venoms are composed
of varying mixtures of both types. Fairley (1929:301) described the constituents
of venom as: (1) neurotoxic elements that act on the bulbar and
spinal ganglion cells of the central nervous system; (2) hemorrhagins that
destroy the lining of the walls of blood vessels; (3) thrombose, producing
clots within blood vessels; (4) hemolysins, destroying red blood corpuscles;
(5) cytolysins that act on leucocytes and on cells of other tissues; (6) elements
that retard coagulation of the blood; (7) antibactericidal substances;
and (8) ferments that prepare food for pancreatic digestion. Elapid snakes
tend to have more of elements 1, 4, and 6 in their venoms, while viperids and
crotalids, of which the cottonmouth is one, have higher quantities of elements
2, 3, and 5. Kellogg (loc. cit.) stated that venom of cottonmouths contains
more neurotoxin than that of rattlesnakes and not only breaks down the nuclei
of ganglion cells but also produces granular disintegration of the myelin sheath
and fragmentation of the conducting portions of nerve fibers.
Thus, venoms contain both toxic elements and non-toxic substances that
promote rapid spreading of the venom through the body of the victim. Jacques
(1956:291) attributed this rapid spreading to the hyaluronidase content of
venoms.
Venom Yield and Toxicity
One of the most important yet undeterminable factors of the gravity of
snakebite is the amount of venom injected into the victim. Since this volume
varies considerably in every bite, attempts have been made to determine the
amount and toxicity of venom produced by each species of poisonous snake.
Individual yield is so variable that a large number of snakes must be milked in
order to determine the average yield. Even then there remains an uncertainty
as to how this amount may compare with that injected by a biting snake.
Wolff and Githens (1939b:234) made 16 venom extractions from a group [474] of cottonmouths in a two-year period. The average yield per snake fluctuated
between 80 and 237 milligrams (actual weight), and toxicity measured as the
minimum lethal dose for pigeons varied from 0.05 to 0.16 milligrams (dry
weight). No decrease in yield or toxicity was evident during this period.
Another group of cottonmouths from which venom was extracted over a
period of five years also showed no decrease in yield or toxicity. Of 315 individual
extractions the average amount obtained from each individual was
0.55 cubic centimeters of liquid or 0.158 grams of dried venom (28.0 per cent
solids). The minimum lethal dosage (M. L. D.) which was determined by
injecting intravenously into 350-gram pigeons was found to be 0.09 milligrams
(dry weight). Each snake carried approximately 1755 M. L. D.'s of venom.
The record venom extraction for the cottonmouth was 4.0 cubic centimeters
(1.094 grams dried venom) taken from a five-foot snake which had been in
captivity for 11 weeks and milked five weeks earlier (Wolff and Githens,
1939a:52). The average yield of venom of cottonmouths is about three times
the average yield reported for copperheads by Fitch (1960:256), a difference
correlated with the greater bulk and relatively large head of the cottonmouth.
Allen and Swindell (1948:13) stated that cottonmouth venom rates third
in potency, compared drop for drop to that of Micrurus fulvius and Crotalus
adamanteus. Freshly dried cottonmouth venom tested on young white rats
showed the lethal dose to be from 23 to 29 milligrams per kilogram of body
weight. The venom of 11 one-week-old cottonmouths was found to be more
potent than that of adult males. Githens (1935:171) rated C. adamanteus venom as being weaker than that of the copperhead (A. contortrix), which he
rated only slightly lower than cottonmouth venom. The crotalids which he
ranked more toxic than cottonmouths are: the Pacific rattlesnake (C. viridis
oreganus) and the massasauga (S. catenatus). He found A. bilineatus, C.
durissus, and C. v. lutosus to have the same toxicity as cottonmouths. Minton
(1953:214) found that the intraperitoneal "lethal dose 50" (the dose capable
of killing half the experimental mice receiving injections of it) was 6.36 milligrams
per kilogram for copperheads. However, in later publications Minton
(1954:1079; 1956:146) reported that the "lethal dose 50" for copperheads was
25.65 milligrams. Approximately the same potency was determined for cottonmouths.
Several rattlesnakes that he tested showed a higher toxicity than
copperheads or cottonmouths.
Criley (1956:378) found the venom of copperheads to be 6.95, nearer
Minton's earlier estimate, and rated cottonmouth venom as being twice as
toxic as that of copperheads. The relative toxicities of other crotalids tested,
considering the cottonmouth to be one unit, were: C. basiliscus, 0.3; A. contortrix,
0.5; C. viridis oreganus, 1.4; A. bilineatus, 2.2; C. adamanteus, 2.3; C. v.
viridis, 3.2; C. durissus terrificus, 27.5.
It can be seen from the above examples that toxicity of venoms and the
resistance of the animal receiving an injection of venom is highly variable.
Possibly the venom of each species of snake has greatest effect on animals of
the particular group relied on for food by the snake. If that is so, the venom
of cottonmouths would be expected to be more toxic when tested on fish,
reptiles, and amphibians than on birds and mammals. Likewise, the venom
of most species of rattlesnakes would be expected to be more virulent when
injected into mammals than when injected into lower vertebrates. But, according
to Netting (1929:108), species of rattlesnakes that prey on cold-blooded [475] animals, which are less susceptible to venoms than warm-blooded
animals, are thought to have highly toxic venoms. This explanation accounts
for the powerful venom of Sistrurus catenatus; and, in this respect, venom of
cottonmouths should be highly toxic also. However, no clear-cut trends have
been shown in most cases. Allen (1937) injected 250-gram guinea pigs with
4 milligrams of venom of various poisonous snakes. Survival time was recorded
in order to indicate the relative potency of the venoms. Of 16 such tests C.
adamanteus held places 1, 2, 3, 12, and 16; Bothrops atrox held places 4, 9,
10, and 13; and A. piscivorus held places 5, 7, 8, and 15. Places 6, 11, and 14
were held by three individuals of different species. No relationship to size
or sex was indicated by the results of this experiment.
Susceptibility of Snakes
Numerous experiments have been conducted to determine the susceptibility
of various snakes to venom. The majority of these experiments were performed
to learn whether or not venomous snakes were immune to their own
poison. Conant (1934:382) reported on a 30-inch cottonmouth that killed
two Pacific rattlesnakes and another cottonmouth. One rattlesnake was bitten
on the tail and the other on or near the head and partially swallowed. Gloyd
(1933:13-14) recorded fatal effects to a rattlesnake from the bite of a cottonmouth.
He also reported on the observations of three other crotalids bitten
by themselves or other snakes, from which no harmful effects were observed.
Allen (1937) injected several snakes with dried cottonmouth venom which
was diluted with distilled water just before each injection. Four cottonmouths
receiving 9, 18, 19, and 20 milligrams of venom per ounce of body weight survived,
while another receiving 18.7 milligrams per ounce died after three hours.
A specimen of S. miliarius receiving 8.3 milligrams per ounce died in about
ten hours, while a C. durissus receiving 12.5 milligrams per ounce succumbed
in 45 minutes. An alligator receiving 6 milligrams per ounce died in 14 hours.
Even the snakes that survived showed some degree of swelling.
The studies of Keegan and Andrews (1942:252) show that king-snakes are
sometimes killed by poisonous snakes. A Lampropeltis calligaster injected with A. contortrix venom (0.767 milligrams per gram) died five days following the
injection. This amount was more than twice the amount of A. piscivorus venom injected into a L. getulus by Allen (1937) in which the snake showed
no ill effects. Keegan and Andrews (loc. cit.) stated that success in overpowering
and eating poisonous snakes by Lampropeltis and Drymarchon may be due
to the ability to avoid bites rather than to immunity to the venom. However,
Rosenfeld and Glass (1940) demonstrated that the plasma of L. g. getulus had an inhibiting effect on the hemorrhagic action on mice of the venoms of
several vipers.
One of the more extensive studies on effects of venoms on snakes is that
by Swanson (1946:242-249). In his studies freshly extracted liquid venom
was used. His studies indicated that snakes are not immune to venom of
their own kind or to closely related species. Copperhead venom killed copperheads
faster than did other venoms but took more time to kill massasaugas,
cottonmouths, and timber rattlers. However, most of the snakes were able to
survive normal or average doses of venom although they are not necessarily
immune to it.
[476]
One of the major problems in comparing the data on toxicity of venom
in studies of this type is that no standard method of estimating toxicity has
been used. Swanson's (loc. cit.) amount of venom equalling one minim
(M.L.D.?) ranged from 0.058 to 0.065 cubic centimeters. There were no
different values given for each species, but the time that elapsed from injection
of the venom to death represented the toxicity. There also was no attempt
in his study to convert the amount of venom used into a ratio of the volume
of venom per weight of snake, making the results somewhat difficult to interpret.
Additional work in this field should provide for many injections into
many individuals of several size classes. The studies to date have been on
far too few individuals to allow statistical analyses to be accurate.
THE BITE
Effects of the Bite
Factors determining the outcome of snakebite are: size, health, and species
of snake; individual variation of venom toxicity of the species; age and size
of the victim; allergic or immune responses; location of the bite; and the
amount of venom injected and the depth to which it is injected. The last
factor is one of the most variable, owing to (1) character and thickness of
clothing between the snake and the victim's skin, (2) accuracy of the snake's
strike, and (3) size of the snake, since a large snake can deliver more venom
and at a greater depth than can a small snake.
Pope and Perkins (1944) demonstrated that pit-vipers of the United States
bite as effectively as most innocuous snakes and that a careful study of the
bite may reveal the location of the pocket of venom, size of the snake, and
possibly its generic identity (see Dentition). The bite pattern of the cottonmouth
as well as the other crotalids showed the typical fang punctures plus
punctures of teeth on both the pterygoid and mandible. Even so, a varying
picture may be presented because from one to four fang marks may be present.
At times in the fang-shedding cycle three and even four fangs can be in
operation simultaneously.
Various authors have attributed death of the prey to the following causes:
paralysis of the central nervous system, paralysis of the respiratory center,
asphyxiation from clotting of the blood, stoppage of the heart, urine suppression
due to crystallized hemoglobin in the kidney tubules, dehydration of
the body following edema in the area of the bite, or tissue damage. Mouths
of snakes are reservoirs for infectious bacteria, which are especially prolific in
damaged tissue. Bacterial growth is aided by the venom which blocks the
bactericidal power of the blood.
Three grades in the severity of snakebite (I, minimal; II, moderate; and
III, severe) were described by Wood, Hoback, and Green (1955). Parrish
(1959:396) added a zero classification to describe the bite of a poisonous
snake in which no envenomation occurred. Grade IV (very severe) was
added by McCollough and Gennaro (1963:961) to account for many bites
of the eastern and western diamondback rattlesnakes.
The first symptom of poisonous snakebite is an immediate burning sensation
at the site of the bite. Within a few minutes the loss of blood into the tissues
causes discoloration. Swelling proceeds rapidly and can become so great as
to rupture the skin. Pain is soon felt in the lymph ducts and glands. Weakness, [477] nausea, and vomiting may ensue at a relatively early stage. Loss of
blood into tissues may spread to the internal organs. In conjunction with a
rapid pulse, the blood pressure and body temperature can drop. Some difficulty
in breathing can occur, especially if large amounts of neurotoxin are
present in the venom. In severe cases the tension due to edema obstructs
venous and even arterial flow, in which case bacteria may multiply rapidly in
the necrotic tissue and gangrene can occur. Blindness due to retinal hemorrhages
may occur. Symptoms of shock may be present after any bite.
Treatment
Perhaps one of the most important factors in the outcome of snakebite is
the treatment. Because of the variable reactions to snakebite, treatment
should vary accordingly. Many methods have been proposed for treating
snakebite, and there is disagreement as to which is the best. The list of
remedies that have been used in cases of snakebite includes many that add
additional injury or that possibly increase the action of the venom. The use
of poultices made by splitting open living chickens and the use of alcohol,
potassium permanganate, strychnine, caffeine, or injection of ammonia have
no known therapeutic value, and may cause serious complications. The most
important steps in the treatment of snakebite are to prevent the spread of
lethal doses of venom, to remove as much venom as possible, and to neutralize
the venom as quickly as possible.
It is generally agreed that the first step in snakebite treatment should be
to place a ligature above the bite to restrict the flow of venom, and also to
immobilize the patient as much as possible. The ligature should be loosened
at least every fifteen minutes. The next steps are sterilization of the skin and
the making of an incision through the fang punctures. As pointed out by
Stahnke (1954:8), the incision should be made in line with the snake's body
at the time of the bite, so as to account for the rearward curvature of the fangs
and possibly to reach the deposition of venom. Many instruction booklets
and first-aid guides have specified the length and depth of incision to be
made, but the actual size and depth of the cut should depend upon the location
of the bite. An "X" cut or connection of the fang punctures is likely to
facilitate the spread of the venom. No cut should be made that would sever
a large blood vessel or ligament.
Extensive damage is often caused by well-meaning individuals whose attempts
at first aid result in brutally deep incisions and tourniquets applied too
tightly and for too long a period of time; the resultant damage in many instances
exceeds that of the bite itself (Stimson and Engelhardt, 1960:165).
Stimson and Engelhardt also think that time should be sacrificed to surgical
cleanliness, and incisions should not be made if a hospital can be reached
within an hour.
The ligature-cryotherapy (L-C) method proposed by Stahnke (1953) has
been severely criticized by other workers. He stated that the ligature should
be tight enough to restrict completely the flow of venom until the temperature
of the area can be lowered sufficiently to prevent any action of the venom.
After 10 minutes the ligature may be removed and the bitten area kept immersed
in a vessel of crushed ice and water. If the envenomized member is
to be treated for more than four hours (which is the case with almost all [478] pit-viper bites), it should be protected by placing it in a plastic bag. The
venom action should be tested after 12 or more hours. This consists of a brief
warming period to determine whether or not the action of the venom can be
felt. The patient should be kept warm at all times; and the warming at the
termination of treatment should be done gradually, preferably by allowing
the water to warm slowly to room temperature.
Advocates of the L-C method warn against making incisions unless they
are absolutely necessary, the theory being that each cut permits additional
bacterial infection and does little good in removing venom. However, McCollough
and Gennaro (1963:963) demonstrated that, in bites where the
fangs had only slightly penetrated the skin, more than 50 per cent of the
venom was removed in some instances if suction was started within three
minutes after the injection. With deeper injection the amount of venom
recovered sometimes reached 20 per cent of the dose. Stahnke suggested that
an incision be made at the site of the bite only after the site has been
refrigerated for at least 30 minutes.
Stimson and Engelhardt (loc. cit.) stated that two constricting bands
should be used between the bite and the body and that cracked ice in a cloth
should be applied to the bite before reaching a hospital. In addition, they
suggested the following procedure. Rings of incisions should follow the swelling,
and suction should continue for several hours. After the edema has
receded, the limb should be wrapped in a towel containing crushed ice.
Antivenin should be given only in severe cases. Calcium gluconate and gas
gangrene antitoxin as well as antibiotics are helpful.
The most recent and up-to-date summary of snakebite treatment is that by
McCollough and Gennaro (1963). Following is a brief summary of their
suggestions:
1. Immobilization—Systemic immobilization is effected by body rest and
locally by splinting the bitten area.
2. Tourniquet—A lightly occlusive tourniquet during a 30- to 60-minute
period of incision and suction would seem to possess some advantages. In
severe cases where medical attention is hours away, a completely occlusive
tourniquet may be necessary to prevent death. Sacrifice of the extremity may
be necessary for the preservation of life.
3. Incision and suction—Suction should begin three to five minutes after
injection of venom if symptoms of poisoning are present. Incisions one-fourth
inch to an inch long across each fang mark should be made in order to open
the wound for more efficient suction. Multiple incisions are not useful for the
removal of venom but may be employed under hospital conditions to reduce
subcutaneous tensions and ischemia.
4. Cryotherapy—An ice cap over the site of the bite for relief of pain
would seem to be permissible, especially prior to the administration of antivenin.
It must be remembered that cooling during the administration of the antivenin
radically reduces the access of the antiserum to the bite area.
5. Antivenin—Antiserum is the keystone to the therapy of snakebite. Careful
evaluation of the severity of the bite and the patient's sensitivity should be
made before the use of antivenin. In Grade II (moderate) bites, the intramuscular
injection on the side of the bite may suffice. In Grades III (severe)
and IV (very severe), shock and systemic effects require intravenous injection.
In bites producing symptoms of this severity, antivenin must be given in [479] amounts large enough to produce clinical improvement. Ten to 20 units may
be necessary to prevent the relapse that sometimes occurs after small doses
of antivenin. Permanent remission of swelling and interruption of necrosis are
the therapeutic end point in the clinical use of the antiserum.
In all cases of snakebite where there is any doubt as to the snake's identity,
it should be killed if possible and taken to the hospital for positive identification.
In many instances of actual bites by poisonous snakes the only treatment
needed was an injection of tetanus antitoxin or toxoid and sedation, because
physical examination revealed no indication of poisoning (Stimson and Engelhardt, loc. cit.).
Case History of a Bite
On July 29, 1963, at 8:20 a.m., I was treating a nine-month-old cottonmouth
for mites. As I dropped the snake into a sink, it twisted its head and
bit the tip of my right middle finger with one fang. The fang entered just
under the fingernail and was directed downward, the venom being injected
about five millimeters below the site of fang penetration. After placing the
snake back in its cage, I squeezed the finger once to promote bleeding, wrapped
a string around the base of the finger, and drove to Watkins Memorial Hospital
on the University of Kansas campus. I began to feel a burning sensation in the
tip of the finger almost immediately. Upon my arrival at the hospital, an additional
ligature was placed around my wrist. At 8:30 a.m. a small incision
was made in the end of the finger, which by this time was beginning to darken
at the point of venom deposition. I sucked on the finger until 8:35 a.m.,
when a pan of ice water that I had requested was brought to me. No pain
was felt except that caused by the ice. Fresh ice was added as needed to
keep the temperature low. By 9:30 a.m. the finger had swollen and stiffened.
At 10:00 a.m. the swelling had progressed to the index finger and back of
the hand. I experienced difficulty in opening and closing the hand. Blood
oozed slowly from the incision. A dull ache persisted and about every two
to four minutes a sharp throb could be felt until nearly 11:00 a.m., when the
pain diminished. The rate and intensity of throbbing increased whenever the
hand was removed from the ice bath for more than a few seconds. Although
only the hand was immersed, the entire forearm was cold. Pain was felt along
the lymphatics on top of the arm when it was touched, and by 1:00 p.m. a
slight pain could be felt in the armpit. Since swelling and pain were almost
nonexistent by 2:00 p.m., I was permitted to leave. After walking to a nearby
building, I again felt a burning sensation as the hand warmed. I made
another ice bath and again immersed the hand in it until 4:10 p.m., at which
time it was removed from the water. The pain and swelling began anew, and
the hand was placed back in an ice bath from 5:30 p.m. until about 7:30 p.m.
At this time cryotherapy was discontinued. From 10:00 p.m. to
12:00 midnight my legs twitched periodically, and pain could be felt in both
armpits. A slight difficulty in breathing also was experienced for a short time.
The acute pain and burning sensation remained in the finger until the following
morning, but swelling progressed only as far as the wrist. The only discomfort
that day was in the finger. The tip was darkened, the entire first
digit red and feverish, and the lymphatics still painful when touched. By the
third day the swelling had regressed. The incision itself was the main cause
of discomfort, and the soreness at the site of the bite persisted for at least four
days.
Although the L-C method of snakebite treatment has been vigorously attacked
by many, there is still need of much more data before it can be unequivocally
condemned or praised. It was preferred in the treatment of this
bite because: I knew that envenomation was minimal and that there would be
no need for antivenin; only one fang of a snake less than one foot long had
entered the tip of the finger; the snake had bitten three frogs in the previous [480] two days and had possibly used up a considerable amount of its venom; the
venom was deposited at such a shallow depth that at least a portion of it
could be removed by suction; and the wound bled freely even before suction
was applied. The ice water was uncomfortably cold but was not cold enough
to cause frostbite, a major objection to the L-C method. Ideally, fresh ice
should be added little by little to replace that which is melting, and the immersed
area should be protected from the water by a plastic bag. Pain and
swelling can be minimized by cryotherapy, but I would recommend its use
only in cases of mild poisoning such as the one described herein.
Snakebite in the United States
Many estimates have been made of the number of bites of poisonous snakes
that occur annually in the United States. The occurrence of poisonous snakebite
has been nearly as badly underestimated as fatal results of their envenomations
have been overrated. For important data on number of persons bitten
by poisonous snakes in the United States, see the following: Allen and
Swindell (1948:15); Githens (1935:172); Klauber (1956:811); Parrish (1963);
Sowder and Gehres (1963:973); Stimson and Engelhardt (1960:153); Swaroop
and Grab (1956:441); Swartzwelder (1950:579); Willson (1908:530); and
Wood (1954b:937).
Judging from estimates made in several states, the number of poisonous
snakebites in the United States would be about 5000 per year. In the region
where the cottonmouth occurs there are approximately 2000 persons bitten
annually by poisonous snakes. Of these approximately 39 per cent are copperhead
bites, 30 per cent each are cottonmouth and rattlesnake bites, and I per
cent are coral snake bites. These percentages vary considerably from place
to place, because of the distribution and abundance of the eight species of
poisonous snakes whose ranges overlap that of the cottonmouth.
According to Parrish (1963), about 14 people die of snakebite each year in
the United States. Of these deaths, about 6.6 per cent are attributable to
cottonmouths, 77.0 per cent to rattlesnakes, and 1.6 per cent to coral snakes;
14.8 per cent are unidentified. Almost half of the fatalities are in persons
less than 20 years of age, the high mortality rate being partially due to the
greater ratio of venom to body weight.
SUMMARY
In my study, 306 living and preserved cottonmouths were examined.
This species occurs throughout the coastal plains of the
southeastern United States, usually at altitudes of less than 500 feet
but occasionally up to altitudes of more than 2000 feet.
Two subspecies are recognized: the eastern cottonmouth, A. p.
piscivorus, occurring from extreme eastern Mississippi to southeastern
Virginia and Florida; and the western cottonmouth, A. p.
leucostoma, occurring from eastern Mississippi northward to southern
Illinois and Missouri and westward to central Texas. Intergradation
occurs in eastern Mississippi.
[481]
The northern edge of the range is probably limited by low temperatures
in winter, and the western edge by lack of available
habitat resulting from insufficient precipitation. Old records of occurrence
indicate that the range has decreased in the last 100 years.
The species inhabits mostly areas where water is found, but at times
wanders a mile or more from the nearest water.
The ground color is predominantly a brown, but varies from a
brownish-green to almost black with a pattern of 10 to 17 irregular
bands of a darker shade of brown. The pattern is better defined in
the eastern subspecies than in the western.
The scutellation resembles that of other species of Agkistrodon.
In the specimens examined supralabials ranged from 7 to 9, and
infralabials from 8 to 12. The number of dorsal scale rows on the
neck, at mid-body, and immediately anterior to the anus is relatively
constant at 27-25-21, respectively. Ventral scales of 34 males averaged
134.4 (128 to 139), and those of 48 females 133.5 (128 to 137).
The number of caudal scales showed some degree of sexual dimorphism;
the average was 45.4 (41 to 50) in 34 males and 42.6
(39 to 49) in 44 females. In general, caudal scales on the basal
half of the tail are undivided, whereas those on the distal half are
divided. No marked geographical variation was found in any scale
character.
The poison fangs vary in length from 1.3 per cent of snout-vent
length in juveniles to 1.0 per cent in large adults. Fangs of
captive cottonmouths were shed and replaced at intervals of about
21 days, but the interval was variable. Relationships in distance
between the base of fangs and between fang punctures in an actual
bite indicate that examination of the wound does not provide a
good basis for judging accurately the size of the snake that inflicted
the bite.
In general, females less than 450 millimeters in snout-vent length
were juveniles; those more than 450 millimeters were classified as
post partum or reproductive on the basis of sizes of ovarian follicles.
Since about half the adult females were fecund, it was concluded
that a biennial reproductive cycle occurs in this species. An annual
cycle may occur in areas where temperature permits year-round
activity. It was estimated that females become sexually mature at
an age of approximately two and one-half years. Mating is probably
most concentrated in early spring at about the time when females
ovulate, but copulation is not a stimulus for ovulation. Sperm
retention and delayed fertilization allow young to be produced without [482] copulation occurring in each breeding season. The testes increase
in size gradually rather than rapidly at maturity or in each
breeding season, but seasonal cycles in sperm production occur.
The gestation period is three and one-half to four months. Determination
of sex in the embryos is possible by late June, because
the hemipenes of males are evaginated until the time of birth.
Parturition generally occurs in August or September, but captivity
may delay birth for a month or more. From one to 16 young per
litter are born, depending on size of the mother and other factors;
but the average is between six and seven. Mortality rate at birth is
high in captive individuals but has not been determined in natural
populations. The sex ratio in embryos and adults examined revealed
about 53 per cent females. Because sufficient information on
population composition is not available, an estimate of the percentage
of adults in a natural population was based upon the number
found in my study. The reproductive potential was estimated
from these figures.
Normal young at birth are 230 to 240 millimeters in snout-vent
length, but their size is influenced by the condition of the mother.
Comparison of newborn young with those captured in spring
indicates that little growth occurs during winter. Early growth is
largely dependent upon feeding before winter quiescence.
The umbilical cord is broken at birth and the navel closes within
a few days, but the scar remains throughout life. Sexual dimorphism
in the position of the scar is characteristic of some snakes but is
minimal in cottonmouths.
In those snakes more than 700 millimeters in length, males outnumber
females three to one. The maximum age of cottonmouths
in nature is unknown, but one has been kept in captivity for more
than 18 years.
Allometric growth is striking in cottonmouths. The head and
tail are proportionately longer in young individuals than in adults;
and in males the tail is, on the average, slightly longer than in
females of the same size.
Shedding of the skin provides for growth and wear in snakes.
The young shed within a few days after birth and generally shed
more frequently than adults. Frequency of shedding depends
mostly on amount of food consumed, and there is some evidence
that injuries on the head and neck increase the frequency of shedding.
Before shedding, the eyes become cloudy for about five [483] and one-half days, then clear up again for about four days before
the skin is shed.
The food of cottonmouths consists mainly of small vertebrates
and occasionally invertebrates that are found near water. Fish,
amphibians, and reptiles make up nearly 70 per cent of the diet.
Carrion is also eaten and cannibalism occurs occasionally. Food
is obtained by lying in ambush or by active searching. The young
are known to lure their prey within striking range by waving their
yellow tails in a manner suggestive of writhing grubs. The method
of obtaining prey differs according to the kind of prey. Generally,
cottonmouths retain their hold on fish or frogs but release mice
and larger prey after delivering a bite.
The major causes of mortality of cottonmouths are obscure.
Predators are known to include alligators, indigo snakes, king-snakes,
largemouth bass, and blue herons; there are probably
numerous others. Heavy parasitic infestations were found among
the snakes examined. Snake mites, Ophionyssus natricus, became
increasingly abundant on almost all captive snakes in April and
May of 1963. Lung flukes (Ochetosoma sp.) were in 16 of 20
captive snakes, and many preserved specimens contained nematodes
(Kalicephalus sp.) in the stomach and/or tapeworms (Ophiotaenia sp.) in the intestine. Although parasitic infestation causes discomfort
and may lower resistance to other detrimental factors, it
is difficult to attribute death to the effect of any particular kind
of parasite. Miscellaneous causes of death of some captive snakes
also were discussed.
The maximal body temperatures tolerated by four cottonmouths
were between 38° and 40° C., but a temperature of 38° was lethal
to a fifth individual. Cottonmouths have been found on occasion
when other snakes were inactive because of low temperatures, but
minimal temperatures tolerated by this species are not known. The
annual cycle of activity is dependent upon temperature and thus
varies from north to south. Cottonmouths generally migrate inland
in autumn, usually to dry forested hillsides, where they den along
with other species of snakes. After a few warm days in spring
they migrate back to the water's edge. The diel activity cycle likewise
depends upon temperatures but is influenced by other factors
as well. In spring and autumn, the snakes are active mostly on
warm, sunny days, whereas in summer they are active mostly at
night. In order to maintain adequate internal temperatures, much [484] time is spent basking mostly in a characteristic flat, resting coil
either beside a body of water or above water on limbs of dead
trees. In this position the snake is ready either for a short strike
or a hasty getaway.
Juveniles appear particularly aggressive and strike repeatedly
when approached, a behavioral pattern definitely favoring survival.
Adults vary in disposition, usually appearing sluggish and lazy,
but they are capable of striking rapidly when disturbed. The typical
threat display consists of lying in a coiled position with the mouth
opened widely, exposing the white interior, and with the tail vibrating
rapidly. The striking posture resembles the resting coil except
that the anterior part of the body is raised off the ground and the
mouth is sometimes opened. Musk is often ejected in a fine spray
from glands in the tail as a further defensive action.
"Head bobbing," more properly described as spastic contractions
of the body, was observed in captives when food was introduced
into a cage containing several individuals or when one of the
snakes was returned to the cage after being handled. Reports in
the literature also have connected these jerking movements with
courtship. The response appears to be elicited whenever a nervous
state is recognized in another individual and may serve to protect
the jerking individual from aggressive advances of the former.
The relatively heavy appearance of the body, sluggish habits,
and cryptic coloration are correlated with the development of
venom and fangs. The poison apparatus has developed primarily
as a means of causing rapid death in prey and secondarily, perhaps,
to begin the digestion of small animals that are the usual prey, but
it is also important as a defensive device. The venom contains at
least eight constituents that aid in its action on prey. Toxicity of
the venom is difficult to determine because of numerous variables,
but cottonmouth venom is generally believed to be less potent
than that of most rattlesnakes and more potent than that of the
copperhead. Snakes in general are more resistant to snake venoms
than other vertebrates of similar size, but there is no immunity
even to their own venom.
About ten per cent of the approximately 5000 bites of poisonous
snakes per year in the United States are attributable to cottonmouths,
and about seven per cent of the approximately 14 deaths
per year are caused by cottonmouths.
[485]
LITERATURE CITED
Allen, E. R.
1937. Florida snake venom experiments. Florida Acad. Sci., 2:7 pp.
Allen, E. R., and Swindell, D.
1948. Cottonmouth moccasin of Florida. Herpetologica, 4:1-16 (first
supplement).
Allen, M. J.
1932. A survey of the amphibians and reptiles of Harrison County, Mississippi.
Amer. Mus. Novit., 542:1-20.
Anderson, P.
1941. The cottonmouth in northern Missouri. Copeia, 1941(3):178.
1945. New herpetological records for Missouri. Bull. Chicago Acad.
Sci., 7(5):271-275.
Anon.
1953. Snake collecting hobby of Colonial Heights lad. Virginia Wildlife,
14-24.
Auffenberg, W.
1963. The fossil snakes of Florida. Tulane Studies Zool., 10(3):131-216,
51 figs.
Bailey, R. M.
1948. Winter mortality in the snake Storeria dekayi. Copeia, 1948(3):
215.
Barbour, R. W.
1956. A study of the cottonmouth, Agkistrodon piscivorus leucostoma Troost, in Kentucky. Trans. Kentucky Acad. Sci., 17(1):33-41,
1 fig.
Blanchard, F. N.
1922. The amphibians and reptiles of western Tennessee. Occ. Pap.
Mus. Zool.; Univ. Michigan, 117:1-18.
Bogert, C. M.
1943. Dentitional phenomena in cobras and other elapids with notes on
adaptive modifications of fangs. Bull. Amer. Mus. Nat. Hist.,
81(3):285-360.
Brattstrom, B. H.
1954. The fossil pitvipers (Reptilia: Crotalidae) of North America.
Trans. San Diego Soc. Nat. Hist., 12(3):31-46, 2 figs.
Brown, A. E.
1903. Texas reptiles and their faunal relations. Proc. Acad. Nat. Sci.
Philadelphia, pp. 543-558.
Carpenter, C. C.
1958. Reproduction, young, eggs, and food of Oklahoma snakes. Herpetologica,
14:113-115.
Carr, A. F., Jr.
1936. The gulf-island cottonmouths. Proc. Florida Acad. Sci., 1:86-90.
Carr, A. F., Jr., and Carr, M. H.
1942. Notes on the courtship of the cottonmouth moccasin. Proc. New
England Zool. Club, July 18, 20:1-6.
Clark, R. F.
1949. Snakes of the hill parishes of Louisiana. Jour. Tennessee Acad.
Sci., 24(4):244-261.
[486]
Conant, R.
1933. Three generations of cottonmouths, Agkistrodon piscivorus (Lacépède).
Copeia, 1933(1):43.1934. Two rattlesnakes killed by a cottonmouth. Science, 80(2078):382.
1958. A field guide to reptiles and amphibians. Houghton Mifflin Co.,
Boston, 366 pp.
Cook, F. A.
1962. Snakes of Mississippi. Mississippi Game and Fish Comm., Surv.
Bull., ii + 45 pp.
Corrington, J. D.
1929. Herpetology of the Columbia, South Carolina region. Copeia,
1929:58-83.
Cowles, R. B.
1938. Unusual defense postures assumed by rattlesnakes. Copeia,
1938(1):13.
Cowles, R. B., and Bogert, C. M.
1944. A preliminary study of the thermal requirements of desert reptiles.
Bull. Amer. Mus. Nat. Hist., 83(5):263-296.
Criley, B. R.
1956. Development of a multivalent antivenin for the family Crotalidae.
Pp. 373-380 in Venoms (ed. Buckley, E. E., and Porges, N., Amer.
Assoc. Adv. Sci., Publ. No. 44).
Darlington, P. J.
1957. Zoogeography: the geographical distribution of animals. John
Wiley and Sons, Inc., New York, xi + 675 pp.
Davis, D. D.
1936. Courtship and mating behavior in snakes. Zool. Series Field Mus.
Nat. Hist., 20:257-290, figs. 28-34.
Didisheim, P., and Lewis, J. H.
1956. Fibrinolytic and coagulent activities of certain snake venoms and
proteases. Proc. Soc. Exp. Biol. Med., 93(1):10-13.
Ditmars, R. L.
1915. The reptile book. Doubleday, Page and Co., Garden City, N. Y.,
xxxii + 472 pp.1945. The reptiles of North America. Doubleday, Doran and Co., Inc.,
Garden City, N. Y., xvi + 476 pp., 135 pls.
Dowling, H. G.
1951a. A proposed standard system of counting ventrals in snakes. British
Jour. Herp., 1(5):97-98, 1 fig.1951b. A proposed method of expressing scale reductions in snakes.
Copeia, 1951(2):131-134.
Dundee, H. A., and Burger, W. L., Jr.
1948. A denning aggregation of the western cottonmouth. Nat. Hist.
Misc., Chicago Acad. Sci., 21:1-2.
Edgren, R. A.
1951. The umbilical scar, a sexually dimorphic character in Heterodon
platyrhinos. Nat. Hist. Misc., 83:1-2.
Fairley, N. H.
1929. The present position of snake bite and the snake bitten in Australia.
Med. Jour. Australia, 1(10):296-313.
[487]
Fitch, H. S.
1956. Temperature responses in free-living amphibians and reptiles in
northeastern Kansas. Univ. Kansas Publ. Mus. Nat. Hist., 8(7):417-476,
10 figs., 6 tables.1960. Autecology of the copperhead. Univ. Kansas Publ. Mus. Nat. Hist.,
13(4):85-288, pls. 13-20, 26 figs.
Force, E. R.
1930. The amphibians and reptiles of Tulsa County, Oklahoma, and
vicinity. Copeia, 1930(2):25-39.
Fox, W.
1948. Effect of temperature on development of scutulation in the garter
snake, Thamnophis elegans atratus. Copeia, 1948(4):252-262.
Githens, T. S.
1935. Studies on the venoms of North American pit vipers. Jour. Immun.,
29(2):165-173.
Glissmeyer, H. R.
1951. Symposium. A snake den in Tooele County, Utah. Egg production
of the great basin rattlesnake. Herpetologica, 7(1):24-27.
Gloyd, H. K.
1933. On the effects of moccasin venom upon a rattlesnake. Science,
78(2010):13-14.1934. Studies on the breeding habits and young of the copperhead, Agkistrodon mokasen Beauvois. Papers Michigan Acad. Sci., Arts
and Letters, 19:587-604.
Gloyd, H. K., and Conant, R.
1943. A synopsis of the American forms of Agkistrodon (copperheads and
moccasins). Bull. Chicago Acad. Sci., 7(2):147-170, 16 figs.
Goodman, J. D.
1958. Material ingested by the cottonmouth, Agkistrodon piscivorus, at
Reelfoot Lake, Tennessee. Copeia, 1958(2):149.
Guidry, E. V.
1953. Herpetological notes from southeastern Texas. Herpetologica, 9:49-56.
Hall, H. H., and Smith, H. M.
1947. Selected records of reptiles and amphibians from southeastern
Kansas. Trans. Kansas Acad. Sci., 49(4):447-454.
Hamilton, W. J., Jr., and Pollack, J. A.
1955. The food of some crotalid snakes from Fort Benning, Georgia.
Nat. Hist. Misc., 140:1-4.
Herald, E. S.
1949. Effects of DDT oil solution upon amphibians and reptiles. Herpetologica,
5(6):117-120.
Hickman, C. P.
1922. A northern record for the water moccasin. Copeia, 1922(106):39.
Hurter, J. H.
1897. A contribution to the herpetology of Missouri. Trans. Acad. Sci.
St. Louis, 7:499-503.
Jacques, R.
1956. The hyaluronidase content of animal venoms. Pp. 291-293 in Venoms (ed. Buckley, E. E., and Porges, N., Amer. Assoc. Adv.
Sci., Publ. No. 44).
[488]
Keegan, H. L., and Andrews, T. F.
1942. Effects of crotalid venom on North American snakes. Copeia,
1942:251-254.
Kellogg, R.
1925. Poisonous snakes of the United States. U. S. D. A. Papers (371),
13 pp.
Klauber, L. M.
1941. Four papers on the application of statistical methods to herpetological
problems. Bull. Zool. Soc. San Diego, 17:1-95.1956. Rattlesnakes. Univ. California Press, 2 vols., xxix + 1476 pp.
Klimstra, W. D.
1959. Food habits of the cottonmouth in southern Illinois. Chicago Acad.
Sci., Nat. Hist. Misc., 168:1-8.
Laughlin, H. E.
1959. Stomach contents of some aquatic snakes from Lake McAlester,
Pittsburgh County, Oklahoma. Texas Jour. Sci., 11(1):83-85.
Lederer, G.
1931. Aus meinem Tagebuch. Wachenschrift fur Aquar. und Terr'kde.,
28:651-653.
Lee, H. T.
1964. Letters to the Editor, Texas Game and Fish Mag., 22(3):32.
Lowe, C. H., Jr.
1948. Territorial behavior in snakes and the so-called courtship dance.
Herpetologica, 4(4):129-145.
Mansueti, R.
1946. Mating of the pilot blacksnake. Herpetologica, 3:98-100, 1 fig.
Martin, J. R., and Wood, J. T.
1955. Notes on the poisonous snakes of the Dismal Swamp area. Herpetologica,
11(3):237-238.
McCollough, N. C., and Gennaro, J. F., Jr.
1963. Evaluation of venomous snake bite in the southern United States
from parallel clinical and laboratory investigations. Jour. Florida
Med. Assn., 49(12):959-967.
McIlhenny, E. A.
1935. The alligator's life history. The Christopher Publ. House, Boston,
Massachusetts, 117 pp.
Minton, S. A., Jr.
1953. Variation in venom samples from copperheads (Agkistrodon contortrix
mokeson) and timber rattlesnakes (Crotalus horridus horridus).
Copeia, 1953:212-215.1954. Polyvalent antivenin in the treatment of experimental snake venom
poisoning. Amer. Jour. Trop. Med. and Hyg., 3:1077-1082.1956. Some properties of North American pit viper venoms and their
correlation with phylogeny. Pp. 145-151 in Venoms (ed. Buckley,
E. E., and Porges, N., Amer. Assoc. Adv. Sci., Publ. No. 44).
Munro, D. F.
1949. Effect of DDT powder on small cottonmouths. Herpetologica,
5:71-72.1950. Additional observations on head bobbing by snakes. Herpetologica,
6:88.
[489]
Neill, W. T.
1947. Size and habits of the cottonmouth moccasin. Herpetologica,
3:203-205.1949. Head bobbing, a widespread habit of snakes. Herpetologica,
5:114-115.1960. The caudal lure of various juvenile snakes. Quart. Jour. Florida
Acad. Sci., 23(3):173-200, 2 figs.
Neill, W. T., and Allen, E. R.
1955. Metachrosis in snakes. Quart. Jour. Florida Acad. Sci., 18(3):207-215.
Netting, M. G.
1929. The venom of Sistrurus catenatus. Bull. Antivenin Inst. Amer.,
2(4):108-109.
Parrish, H. M.
1959. Poisonous snakebites resulting in lack of venom poisoning. Virginia
Med. Month, 86:396-401.1963. Analysis of 460 fatalities from venomous animals in the United
States. Amer. Jour. Med. Sci., 245(2):35-47.
Parrish, H. M., and Pollard, C. B.
1959. Effects of repeated poisonous snakebite in man. Amer. Jour. Med.
Sci., 237(3):277-286.
Penn, G. H.
1943. Herpetological notes from Cameron Parish, Louisiana. Copeia,
1943(1):58-59.
Perkins, C. B.
1955. Longevity of snakes in captivity in the United States. Copeia,
1955(3):262.
Pope, C. H., and Perkins, R. M.
1944. Differences in the patterns of bites of venomous and of harmless
snakes. Archives of Surgery, 49:331-336.
Rahn, H.
1942. The reproductive cycle of the prairie rattler. Copeia, 1942(4):233-240.
Ramsey, L. W.
1948. Combat dance and range extension of Agkistrodon piscivorus
leucostoma. Herpetologica, 4:228.
Richmond, M. D.
1952. Head bobbing in reptiles. Herpetologica, 8:38.
Rosenfeld, S., and Glass, S.
1940. The inhibiting effect of snake bloods upon the hemorrhagic action
of viper venoms on mice. Amer. Jour. Med. Sci., 199:482-486.
Schmidt, K. P.
1946. On the zoogeography of the Holarctic region. Copeia, 1946:144-152.
Shaw, C. E.
1948. The male combat "dance" of some crotalid snakes. Herpetologica,
4:137-145.1951. Male combat in American colubrid snakes with remarks on combat
in other colubrid and elapid snakes. Herpetologica, 7(4):149-168.
Smith, H. M.
1956. Handbook of amphibians and reptiles of Kansas. 2nd Edition.
Univ. Kansas Mus. Nat. Hist., Misc. Publ., 9:1-356, 253 figs.
[490]
Smith, H. M., and Buechner, H. K.
1947. The influence of the Balcones Escarpment on the distribution of
amphibians and reptiles in Texas. Bull. Chicago Acad. Sci.,
pp. 1-16.
Smith, P. W.
1961. The amphibians and reptiles of Illinois. Illinois Nat. Hist. Survey,
28(1):1-298.
Smith, P. W., and List, J. C.
1955. Notes on Mississippi amphibians and reptiles. Amer. Midl. Nat.,
53(1):115-125.
Sowder, W. T., and Gehres, G. W.
1963. Snakebites in Florida. Jour. Florida Med. Assn., 49(12):973-976.
Stabler, R. M.
1951. Some observations on two cottonmouth moccasins made during 12
and 14 years of captivity. Herpetologica, 7:89-92.
Stahnke, H. M.
1953. The L-C treatment of venomous bites and stings. Amer. Jour.
Trop. Med. and Hyg., 2(1):142-143.1954. The L-C method of treating venomous bites and stings. Pois.
Anim. Res. Lab., Arizona State Coll., 28 pp.
Stejneger, L.
1895. The poisonous snakes of North America. Smithsonian Inst., U. S.
Nat. Mus., 1893:337-487, pls. 1-19, figs. 1-70.
Stimson, A. C., and Engelhardt, T. H.
1960. The treatment of snakebite. Jour. Occ. Med., 2(4):163-168.
Sutherland, I. D.
1958. The "combat dance" of the timber rattlesnake. Herpetologica,
14(1):23-24.
Swanson, P. L.
1946. Effects of snake venoms on snakes. Copeia, 1946(4):242-249.
Swaroop, S., and Grab, B.
1956. The snakebite mortality problem in the world. Pp. 439-466 in Venoms (ed. Buckley, E. E., and Porges, N., Amer. Assoc. Adv.
Sci., Publ. No. 44).
Swartzwelder, J. C.
1950. Snake-bite accidents in Louisiana with data on 306 cases. Amer.
Jour. Trop. Med., 30(4):575-589.
Tinkle, D. W.
1962. Reproductive potential and cycles in female Crotalus atrox from
northwestern Texas. Copeia, 1962(2):306-313.
Trowbridge, A. H.
1937. Ecological observations on amphibians and reptiles collected in
southeastern Oklahoma during the summer of 1934. Amer. Midl.
Nat., 18(2):285-303.
Volsøe, H.
1944. Structure and seasonal variation of the male reproductive organs
in Vipera berus (L.). Spolia Zool. Mus. Hauniensis V. Reprint,
Copenhagen, pp. 1-172.
Wharton, C. H.
1960. Birth and behavior of a brood of cottonmouths, Agkistrodon piscivorus
piscivorus, with notes on tail-luring. Herpetologica, 16:125-129.
[491]
Willson, P.
1908. Snake poisoning in the United States: a study based on an
analysis of 740 cases. Arch. Int. Med., 1(5):516-570.
Wolff, N. O., and Githens, T. S.
1939a. Record venom extraction from water moccasin. Copeia, 1939(1):52.
1939b. Yield and toxicity of venom from snakes extracted over a period of
two years. Copeia, 1939(4):234.
Wood, J. T.
1954a. The distribution of poisonous snakes in Virginia. Virginia Jour.
Sci., 5(3):152-167, 4 maps.1954b. A survey of 200 cases of snake-bite in Virginia. Amer. Jour. Trop.
Med. and Hyg., 3(5):936-943.
Wood, J. T., Hoback, W. W., and Green, T. W.
1955. Treatment of snake venom poisoning with ACTH and cortisone.
Virginia Med. Month, 82:130-135.
Wright, A. H., and Wright, A. A.
1957. Handbook of snakes of the United States and Canada. Comstock
Publ. Assoc., Cornell Univ. Press, 2:ix + 565-1106 pp.
Yamaguti, S.
1958. Systema helminthum. Interscience Publ., Inc., New York, 3 vols.,
5 parts, 1:xi + 1575 pp., 2:vii + 860 pp., 3:1261 pp.
Yerger, R. W.
1953. Yellow bullhead preyed upon by cottonmouth moccasin. Copeia,
1953(2):115.
Transmitted June 20, 1966.
UNIVERSITY OF KANSAS PUBLICATIONS
MUSEUM OF NATURAL HISTORY
Institutional libraries interested in publications exchange may obtain this
series by addressing the Exchange Librarian, University of Kansas Library,
Lawrence, Kansas. Copies for individuals, persons working in a particular
field of study, may be obtained by addressing instead the Museum of Natural
History, University of Kansas, Lawrence, Kansas. When copies are requested
from the Museum, 25 cents should be included (for each 100 pages or part
thereof) for the purpose of defraying the costs of wrapping and mailing. For
certain longer papers an additional amount indicated below, toward the cost
of production, is to be included. Materials published to date in this series
are as follows.
* An asterisk designates those numbers of which the Museum's supply (not necessarily
the Library's supply) is exhausted. Materials published to date, in this series, are as
follows:
Vol. 1. Nos. 1-26 and index. Pp. 1-638, 1946-1950.
*Vol. 2. (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140 figures in text. April 9, 1948.
*Vol. 3. Nos. 1-4 and index. Pp. 1-681. 1951.
*Vol. 4. (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31 figures in text. December 27, 1951.
Vol. 5. Nos. 1-37 and index. Pp. 1-676, 1951-1953.
*Vol. 6. (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D. Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952.
Vol. 7. Nos. 1-15 and index. Pp. 1-651, 1952-1955.
Vol. 8. Nos. 1-10 and index. Pp. 1-675, 1954-1956.
Vol. 9. Nos. 1-23 and index. Pp. 1-690, 1955-1960.
Vol. 10. Nos. 1-10 and index. Pp. 1-626, 1956-1960.
Vol. 11. Nos. 1-10 and index. Pp. 1-703, 1958-1960.
Vol. 12. *1. Functional morphology of three bats: Eumops, Myotis, Macrotus. By Terry
A. Vaughan. Pp. 1-153, 4 plates, 24 figures in text. July 8, 1959.
*2. The ancestry of modern Amphibia: a review of the evidence. By Theodore
H. Eaton, Jr. Pp. 155-180, 10 figures in text. July 10, 1959.
3. The baculum in microtine rodents. By Sydney Anderson. Pp. 181-216, 49
figures in text. February 19, 1960.
*4. A new order of fishlike Amphibia from the Pennsylvanian of Kansas. By
Theodore H. Eaton, Jr., and Peggy Lou Stewart. Pp. 217-240, 12 figures in
text. May 2, 1960.
5. Natural history of the Bell Vireo. By Jon C. Barlow. Pp. 241-296, 6 figures
in text. March 7, 1962.
6. Two new pelycosaurs from the lower Permian of Oklahoma. By Richard C.
Fox. Pp. 297-307, 6 figures in text. May 21, 1962.
7. Vertebrates from the barrier island of Tamaulipas, México. By Robert K.
Selander, Richard F. Johnston, B. J. Wilks, and Gerald G. Raun. Pp. 309-345,
plates 5-8. June 18, 1962.
8. Teeth of edestid sharks. By Theodore H. Eaton, Jr. Pp. 347-362, 10 figures
in text. October 1, 1962.
9. Variation in the muscles and nerves of the leg in two genera of grouse
(Tympanuchus and Pedioecetes). By E. Bruce Holmes. Pp. 363-474, 20
figures. October 25, 1962. $1.00.
10. A new genus of Pennsylvanian fish (Crossopterygii, Coelacanthiformes) from
Kansas. By Joan Echols. Pp. 475-501, 7 figures. October 25, 1963.
11. Observations on the Mississippi Kite in southwestern Kansas. By Henry S.
Fitch. Pp. 503-519. October 25, 1963.
12. Jaw musculature of the Mourning and White-winged doves. By Robert L.
Merz. Pp. 521-551, 22 figures. October 25, 1963.
13. Thoracic and coracoid arteries in two families of birds, Columbidae and
Hirundinidae. By Marion Anne Jenkinson. Pp. 553-573, 7 figures. March
2, 1964.
14. The breeding birds of Kansas. By Richard F. Johnston. Pp. 575-655, 10
figures. May 18, 1964. 75 cents.
15. The adductor muscles of the jaw in some primitive reptiles. By Richard C.
Fox. Pp. 657-680, 11 figures in text. May 18, 1964.
Index. Pp. 681-694.
Vol. 13. 1. Five natural hybrid combinations in minnows (Cyprinidae). By Frank B.
Cross and W. L. Minckley. Pp. 1-18. June 1, 1960.
2. A distributional study of the amphibians of the Isthmus of Tehuantepec,
México. By William E. Duellman. Pp. 19-72, plates 1-8, 3 figures in text.
August 16, 1960. 50 cents.
3. A new subspecies of the slider turtle (Pseudemys scripta) from Coahuila,
México. By John M. Legler. Pp. 73-84, plates 9-12, 3 figures in text.
August 16, 1960.
*4. Autecology of the copperhead. By Henry S. Fitch. Pp. 85-288, plates 13-20,
26 figures in text. November 30, 1960.
5. Occurrence of the garter snake, Thamnophis sirtalis, in the Great Plains and
Rocky Mountains. By Henry S. Fitch and T. Paul Maslin. Pp. 289-308,
4 figures in text. February 10, 1961.
6. Fishes of the Wakarusa River in Kansas. By James E. Deacon and Artie L.
Metcalf. Pp. 309-322, 1 figure in text. February 10, 1961.
7. Geographic variation in the North American cyprinid fish, Hybopsis gracilis.
By Leonard J. Olund and Frank B. Cross. Pp. 323-348, plates 21-24, 2
figures in text. February 10, 1961.
8. Descriptions of two species of frogs, genus Ptychohyla; studies of American
hylid frogs, V. By William E. Duellman. Pp. 349-357, plate 25, 2
figures in text. April 27, 1961.
9. Fish populations, following a drought, in the Neosho and Marais des Cygnes
rivers of Kansas. By James Everett Deacon. Pp. 359-427, plates 26-30,
3 figures. August 11, 1961. 75 cents.
10. Recent soft-shelled turtles of North America (family Trionychidae). By
Robert G. Webb. Pp. 429-611, plates 31-54, 24 figures in text. February
16, 1962. $2.00.
Index. Pp. 613-624.
Vol. 14. 1. Neotropical bats from western México. By Sydney Anderson. Pp. 1-8.
October 24, 1960.
2. Geographic variation in the harvest mouse, Reithrodontomys megalotis, on
the central Great Plains and in adjacent regions. By J. Knox Jones, Jr.,
and B. Mursaloglu. Pp. 9-27, 1 figure in text. July 24, 1961.
3. Mammals of Mesa Verde National Pork, Colorado. By Sydney Anderson.
Pp. 29-67, plates 1 and 2, 3 figures in text. July 24, 1961.
4. A new subspecies of the black myotis (bat) from eastern Mexico. By E.
Raymond Hall and Ticul Alvarez. Pp. 69-72, 1 figure in text. December
29, 1961.
5. North American yellow bats, "Dasypterus," and a list of the named kinds
of the genus Lasiurus Gray. By E. Raymond Hall and J. Knox Jones, Jr.
Pp. 73-98, 4 figures in text. December 29, 1961.
6. Natural history of the brush mouse (Peromyscus boylii) in Kansas with
description of a new subspecies. By Charles A. Long. Pp. 99-111, 1 figure
in text. December 29, 1961.
7. Taxonomic status of some mice of the Peromyscus boylii group in eastern
Mexico, with description of a new subspecies. By Ticul Alvarez. Pp. 113-120,
1 figure in text. December 29, 1961.
8. A new subspecies of ground squirrel (Spermophilus spilosoma) from Tamaulipas,
Mexico. By Ticul Alvarez. Pp. 121-124. March 7, 1962.
9. Taxonomic status of the free-tailed bat, Tadarida yucatanica Miller. By J.
Knox Jones, Jr., and Ticul Alvarez. Pp. 125-133, 1 figure in text. March 7,
1962.
10. A new doglike carnivore, genus Cynaretus, from the Clarendonian Pliocene,
of Texas. By E. Raymond Hall and Walter W. Dalquest. Pp. 135-138,
2 figures in text. April 30, 1962.
11. A new subspecies of wood rat (Neotoma) from northeastern Mexico. By
Ticul Alvarez. Pp. 139-143, April 30, 1962.
12. Noteworthy mammals from Sinaloa, Mexico. By J. Knox Jones, Jr., Ticul
Alvarez, and M. Raymond Lee. Pp. 145-159. 1 figure in text. May 18,
1962.
13. A new bat (Myotis) from Mexico. By E. Raymond Hall. Pp. 161-164,
1 figure in text. May 21, 1962.
*14. The mammals of Veracruz. By E. Raymond Hall and Walter W. Dalquest.
Pp. 165-362, 2 figures. May 20, 1963. $2.00.
15. The recent mammals of Tamaulipas, México. By Ticul Alvarez. Pp. 363-473,
5 figures in text. May 20, 1963. $1.00.
16. A new subspecies of the fruit-eating bat, Sturnira ludovici, from western
Mexico. By J. Knox Jones, Jr., and Gary L. Phillips. Pp. 475-481, 1 figure
in text. March 2, 1964.
17. Records of the fossil mammal Sinclairella, Family Apatemyidae, from the
Chadronian and Orellan. By William A. Clemens. Pp. 483-491. 2 figures
in text. March 2, 1964.
18. The mammals of Wyoming. By Charles A. Long. Pp. 493-758, 82 figs.
July 6, 1965. $3.00.
Index. Pp. 759-784.
Vol. 15. 1. The amphibians and reptiles of Michoacán, México. By William E. Duellman.
Pp. 1-148, plates 1-6, 11 figures in text. December 20, 1961. $1.50.
2. Some reptiles and amphibians from Korea. By Robert G. Webb, J. Knox
Jones, Jr., and George W. Byers. Pp. 149-173. January 31, 1962.
3. A new species of frog (Genus Tomodactylus) from western México. By
Robert G. Webb. Pp. 175-181, 1 figure in text. March 7, 1962.
4. Type specimens of amphibians and reptiles in the Museum of Natural History,
the University of Kansas. By William E. Duellman and Barbara Berg.
Pp. 183-204. October 26, 1962.
5. Amphibians and Reptiles of the Rainforests of Southern El Petén, Guatemala.
By William E. Duellman. Pp. 205-249, plates 7-10, 6 figures in text. October
4, 1963.
6. A revision of snakes of the genus Conophis (Family Colubridae, from Middle
America). By John Wellman. Pp. 251-295, 9 figures in text. October 4,
1963.
7. A review of the Middle American tree frogs of the genus Ptychohyla. By
William E. Duellman. Pp. 297-349, plates 11-18, 7 figures in text. October
18, 1963. 50 cents.
*8. Natural history of the racer Coluber constrictor. By Henry S. Fitch. Pp.
351-468, plates 19-22, 20 figures in text. December 30, 1963. $1.00.
9. A review of the frogs of the Hyla bistincta group. By William E. Duellman.
Pp. 469-491, 4 figures in text. March 2, 1964.
10. An ecological study of the garter snake, Thamnophis sirtalis. By Henry S.
Fitch. Pp. 493-564, plates 23-25, 14 figures in text. May 17, 1965.
11. Breeding cycle in the ground skink, Lygosoma laterale. By Henry S. Fitch
and Harry W. Greene. Pp. 565-575, 3 figures in text. May 17, 1965.
12. Amphibians and reptiles from the Yucatan Peninsula, México. By William
E. Duellman. Pp. 577-614, 1 figure in text. June 22, 1965.
13. A new species of turtle, Genus Kinosternon, from Central America, by John
M. Legler. Pp. 615-625, pls. 26-28, 2 figures in text. July 20, 1965.
14. A biogeographic account of the herpetofauna of Michoacán, México. By
William E. Duellman. Pp. 627-709, pls. 29-36, 5 figures in text. December
30, 1965.
15. Amphibians and reptiles of Mesa Verde National Park, Colorado. By Charles
L. Douglas. Pp. 711-744, pls. 37, 38, 6 figures in text. March 7, 1966.
Index. Pp. 745-770.
Vol. 16. 1. Distribution and taxonomy of Mammals of Nebraska. By J. Knox Jones, Jr.
Pp. 1-356, pls. 1-4, 82 figures in text. October 1, 1964. $3.50.
2. Synopsis of the lagomorphs and rodents of Korea. By J. Knox Jones, Jr.,
and David H. Johnson. Pp. 357-407. February 12, 1965.
3. Mammals from Isla Cozumel, Mexico, with description of a new species of
harvest mouse. By J. Knox Jones, Jr., and Timothy E. Lawlor. Pp. 409-419,
1 figure in text. April 13, 1965.
4. The Yucatan deer mouse, Peromyscus yucatanicus. By Timothy E. Lawlor.
Pp. 421-438, 2 figures in text. July 20, 1965.
5. Bats from Guatemala. By J. Knox Jones, Jr. Pp. 439-472. April 18, 1966.
More numbers will appear in volume 16.
Vol. 17. 1. Localities of fossil vertebrates obtained from the Niobrara Formation (Cretaceous)
of Kansas. By David Bardack. Pp. 1-14. January 22, 1965.
2. Chorda tympani branch of the facial nerve in the middle ear of tetrapods.
By Richard C. Fox. Pp. 15-21, May 22, 1965.
3. Fishes of the Kansas River System in relation to zoogeography of the Great
Plains. By Artie L. Metcalf. Pp. 23-189, 4 figures in text, 51 maps.
March 24, 1966.
4. Factors affecting growth and reproduction of channel catfish, Ictalurus punctatus.
By Bill A. Simco and Frank B. Cross. Pp. 191-256, 13 figures in
text. June 6, 1966.
5. A new species of fringe-limbed tree frog, genus Hyla, from Darién, Panamá.
By William E. Duellman. Pp. 257-262, 1 figure in text. June 17, 1966.
6. Taxonomic notes on some Mexican and Central American hylid frogs. By
William E. Duellman. Pp. 263-279. June 17, 1966.
7. Neotropical hylid frogs, genus Smilisca. By William E. Duellman and
Linda Trueb. Pp. 281-375, pls. 1-12, 17 figures in text. July 14, 1966.
8. Birds from North Borneo. By Max C. Thompson. Pp. 377-433, 1 figure in
text. October 27, 1966.
9. Natural history of cottonmouth moccasin, Agkistrodon piscivorus (Reptilia).
By Ray D. Burkett. Pp. 435-491, 7 figures in text. October 27, 1966.
More numbers will appear in volume 17.
Featured Books

No Hero
E. W. Hornung
ant grace quite foreign to his ordinary caligraphy.These reflections, trite enough as I know, are ne...

Jaw Musculature of the Mourning and White-winged Doves
Robert L. Merz
at,together with the results of other studies, the relationships of thegenera Zenaida and Zenaidura ...

Kim
Rudyard Kipling
’s family and had married KimballO’Hara, a young colour-sergeant of the Mavericks, an Irish regi...

The Eagle Cliff
R. M. Ballantyne
as, with legs over the handles, he flashed—if he did not actually fly—down one of our Middlesex ...

Fort Desolation: Red Indians and Fur Traders of Rupert's Land
R. M. Ballantyne
arge class. He was what we may call an outskirter of the world. He was one of those who, from the fo...

At Aboukir and Acre: A Story of Napoleon's Invasion of Egypt
G. A. Henty
stupendous one, and thewarlike chiefs of Northern India, who, as yet, were not even threatenedby a B...

Search the Sky
Frederik Pohl and C. M. Kornbluth
not since childhood. Ghost Townwas a wonderful place to play. “Tag,” “Follow My Fuehrer,”�...

Ask a Foolish Question
Robert Sheckley
use Answerer knows everything.Upon his planet, circling his sun, Answerer sat. Duration continued,lo...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "Natural History of Cottonmouth Moccasin, Agkistrodon piscovorus (Reptilia)" :