The Project Gutenberg eBook of The Adductor Muscles of the Jaw In Some Primitive Reptiles
most other parts of the world at no cost and with almost no restrictions
whatsoever. You may copy it, give it away or re-use it under the terms
of the Project Gutenberg License included with this ebook or online
at www.gutenberg.org. If you are not located in the United States,
you will have to check the laws of the country where you are located
before using this eBook.
Title: The Adductor Muscles of the Jaw In Some Primitive Reptiles
Author: Richard C. Fox
Release date: October 24, 2009 [eBook #30321]
Most recently updated: October 24, 2024
Language: English
Credits: Produced by Chris Curnow, Joseph Cooper, Diane Monico, and
the Online Distributed Proofreading Team at
https://www.pgdp.net
*** START OF THE PROJECT GUTENBERG EBOOK THE ADDUCTOR MUSCLES OF THE JAW IN SOME PRIMITIVE REPTILES ***
University of Kansas Publications
Museum of Natural History
Volume 12, No. 15, pp. 657-680, 11 figs.
May 18, 1964
The Adductor Muscles of the Jaw
In Some Primitive Reptiles
BY
RICHARD C. FOX
University of Kansas
Lawrence
1964
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, Henry S. Fitch,
Theodore H. Eaton, Jr.
Volume 12, No. 15, pp. 657-680, 11 figs.
Published May 18, 1964
University of Kansas
Lawrence, Kansas
PRINTED BY
HARRY (BUD) TIMBERLAKE, STATE PRINTER
TOPEKA, KANSAS
1964
30-1522
[Pg 659]
The Adductor Muscles of the Jaw
In Some Primitive Reptiles
BY
RICHARD C. FOX
Information about osteological changes in the groups of reptiles
that gave rise to mammals is preserved in the fossil record, but the
musculature of these reptiles has been lost forever. Nevertheless,
a reasonably accurate picture of the morphology and the spatial
relationships of the muscles of many of these extinct vertebrates
can be inferred by studying the scars or other marks delimiting the
origins and insertions of muscles on the skeletons of the fossils and
by studying the anatomy of Recent genera. A reconstruction built
by these methods is largely speculative, especially when the fossil
groups are far removed in time, kinship and morphology from
Recent kinds, and when distortion, crushing, fragmentation and
overzealous preparation have damaged the surfaces associated with
the attachment of muscles. The frequent inadequacy of such direct
evidence can be partially offset by considering the mechanical demands
that groups of muscles must meet to perform a particular
movement of a skeletal member.
Both direct anatomical evidence and inferred functional relations
were used to satisfy the purposes of the study here reported
on. The following account reports the results of my efforts to: 1,
reconstruct the adductor muscles of the mandible in Captorhinus
and Dimetrodon; 2, reconstruct the external adductors of the mandible
in the cynodont Thrinaxodon; and 3, learn the causes of the
appearance and continued expansion of the temporal fenestrae
among the reptilian ancestors of mammals.
The osteology of these three genera is comparatively well-known.
Although each of the genera is somewhat specialized, none seems
to have departed radically from its relatives that comprised the
line leading to mammals.
I thank Prof. Theodore H. Eaton, Jr., for suggesting the study
here reported on, for his perceptive criticisms regarding it, and for
his continued patience throughout my investigation. Financial assistance
was furnished by his National Science Foundation Grant
(NSF-G8624) for which I am also appreciative. I thank Dr. Rainer
Zangerl, Chief Curator of Geology, Chicago Museum of Natural
History, for permission to examine the specimens of Captorhinus[Pg 660]
and Dimetrodon in that institution. I am grateful to Mr. Robert
F. Clarke, Assistant Professor of Biology, The Kansas State Teachers
College, Emporia, Kansas, for the opportunity to study his specimens
of Captorhinus from Richard's Spur, Oklahoma. Special
acknowledgment is due Mr. Merton C. Bowman for his able preparation
of the illustrations.
Captorhinus
The outlines of the skulls of Captorhinus differ considerably from
those of the skulls of the primitive captorhinomorph Protorothyris.
Watson (1954:335, Fig. 9) has shown that in the morphological
sequence, Protorothyris—Romeria—Captorhinus, there has been
flattening and rounding of the skull-roof and loss of the primitive
"square-cut" appearance in transverse section. The quadrates in
Captorhinus are farther from the midline than in Protorothyris, and
the adductor chambers in Captorhinus are considerably wider than
they were primitively. Additionally, the postorbital region of Captorhinus
is relatively longer than that of Protorothyris, a specialization
that has increased the length of the chambers within.
In contrast with these dimensional changes there has been little
shift in the pattern of the dermal bones that roof the adductor
chambers. The most conspicuous modification in Captorhinus is
the absence of the tabular. This element in Protorothyris was limited
to the occiput and rested without sutural attachment upon the
squamosal (Watson, 1954:338); later loss of the tabular could have
had no effect upon the origins of muscles from inside the skull roof.
Changes in pattern that may have modified the origin of the adductors
in Captorhinus were correlated with the increase in length
of the parietals and the reduction of the supratemporals. Other
changes that were related to the departure from the primitive
romeriid condition of the adductors included the development of
a coronoid process, the flattening of the quadrate-articular joint,
and the development of the peculiar dentition of Captorhinus.
The adductor chambers of Captorhinus are large. They are covered
dorsally and laterally by the parietal, squamosal, postfrontal,
postorbital, quadratojugal and jugal bones. The chamber extends
medially to the braincase, but is not limited anteriorly by a bony
wall. The occiput provides the posterior limit. The greater part
of the adductor chambers lies mediad of the mandibles and thus
of the Meckelian fossae; consequently the muscles that arise from
the dermal roof pass downward and outward to their insertion on
the mandibular rami.
[Pg 661]
Mandible
The mandibular rami of Captorhinus are strongly constructed.
Each ramus is slightly convex in lateral outline. Approximately the
anterior half of each ramus lies beneath the tooth-row. This half
is roughly wedge-shaped in its lateral aspect, reaching its greatest
height beneath the short posterior teeth.
The posterior half of each ramus is not directly involved in supporting
the teeth, but is associated with the adductor musculature
and the articulation of the ramus with the quadrate. The ventral
margin of this part of the ramus curves dorsally in a gentle arc
that terminates posteriorly at the base of the retroarticular process.
The dorsal margin in contrast sweeps sharply upward behind the
teeth and continues posteriorly in a long, low, truncated coronoid
process.
A prominent coronoid process is not found among the more
primitive members of the suborder, such as Limnoscelis, although
the mandible commonly curves upward behind the tooth-row in
that genus. This area in Limnoscelis is overlapped by the cheek
when the jaw is fully adducted (Romer, 1956:494, Fig. 213), thereby
foreshadowing the more extreme condition in Captorhinus.
The coronoid process in Captorhinus is not oriented vertically,
but slopes inward toward the midline at approximately 45 degrees,
effectively roofing the Meckelian fossa and limiting its opening to
the median surface of each ramus. When the jaw was adducted,
the coronoid process moved upward and inside the cheek. A space
persisted between the process and the cheek because the process
sloped obliquely away from the cheek and toward the midline of
the skull. The external surface of the process presented an area
of attachment for muscles arising from the apposing internal surface
of the cheek.
Palate
The palate of Captorhinus is of the generalized rhynchocephalian
type (Romer, 1956:71). In Captorhinus the pterygoids and palatines
are markedly arched and the relatively large pterygoid flange
lies almost entirely below the lower border of the cheek. The
lateral edge of the flange passes obliquely across the anterior lip
of the Meckelian fossa and abuts against the bottom lip of the fossa
when the jaw is closed.
The palatines articulate laterally with the maxillary bones by
means of a groove that fits over a maxillary ridge. This presumably
allowed the halves of the palate to move up and down rather freely.
The greatest amplitude of movement was at the midline. Anteroposterior[Pg 662]
sliding of the palate seems impossible in view of the firm
palatoquadrate and quadrate-quadratojugal articulations.
The subtemporal fossa is essentially triangular, and its broad
end is bounded anteriorly by the pterygoid flange. The fossa is
lateral to much of the adductor chamber; consequently muscles
arising from the parietals passed ventrolaterally, parallel to the
oblique quadrate ramus of the pterygoid, to their attachment on
the mandible.
Musculature
These osteological features indicate that the adductor muscles
of the jaw in Captorhinus consisted of two primary masses (Figs. 1,
2, 3). The first of these, the capitimandibularis, arose from the
internal surface of the cheek and roof of the skull and inserted on
the bones of the lower jaw that form the Meckelian canal and the
coronoid process.
Fig. 1. Captorhinus. Internal aspect of skull, showing
masseter, medial adductor, and temporal muscles. Unnumbered
specimen, coll. of Robert F. Clarke. Richard's Spur, Oklahoma. × 2.
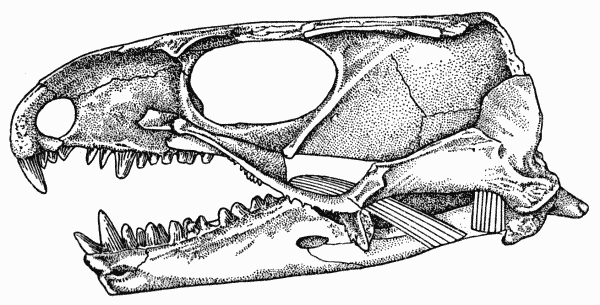
Fig. 2. Captorhinus. Internal aspect of skull, showing anterior
and posterior pterygoid muscles. Same specimen shown in Fig. 1. × 2.
The muscle was probably divided into a major medial mass, the
temporal, and a lesser, sheetlike lateral mass, the masseter. The[Pg 663]
temporal was the largest of the adductors and arose from the lateral
parts of the parietal, the dorsal parts of the postorbital, the most
posterior extent of the postfrontal, and the upper parts of the
squamosal. The muscle may have been further subdivided, but
evidence for subordinate slips is lacking. The fibers of this mass
were nearly vertically oriented in lateral aspect since the parts of
the ramus that are available for their insertion lie within the anteroposterior
extent of the adductor chamber. In anterior aspect the
fibers were obliquely oriented, since the jaw and subtemporal fossa
are lateral to much of the skull-roof from which the fibers arose.
The masseter probably arose from the quadratojugal, the jugal,
and ventral parts of the squamosal, although scars on the quadratojugal
and jugal are lacking. The squamosal bears an indistinct,
gently curved ridge, passing upward and forward from the posteroventral
corner of the bone and paralleling the articulation of the
squamosal with the parietal. This ridge presumably marks the
upper limits of the origin of the masseter from the squamosal.

Fig. 3. Captorhinus. Cross-section of right half of skull immediately
behind the pterygoid flange, showing masseter, temporal, and anterior pterygoid muscles.
Same specimen shown in Fig. 1. × 2.

Fig. 4. Captorhinus. Internal aspect of left mandibular
fragment, showing insertion of posterior pterygoid muscle.
KU 8963, Richard's Spur, Oklahoma. × 2.8.
[Pg 664]
The masseter inserted on the external surface of the coronoid
process, within two shallow concavities separated by an oblique
ridge. The concavities and ridge may indicate that the muscle
was divided into two sheets. If so, the anterior component was
wedge-shaped in cross-section, and its thin posterior edge overlapped
the larger mass that inserted on the posterior half of the
coronoid process.
From a functional standpoint it is doubtful that a major component
of the adductors arose from the quadrate wing of the
pterygoid, for when the jaw is closed the Meckelian fossa is directly
lateral to that bone. If the jaw were at almost any angle but maximum
depression, the greatest component of force would be mediad,
pulling the rami together and not upward. The mediad component
would increase as the jaw approached full adduction. Neither is
there anatomical evidence for an adductor arising from the quadrate
wing of the pterygoid. The bone is smooth, hard, and without
any marks that might be interpreted as muscle scars.
The internal adductor or pterygoid musculature in Captorhinus
consisted of anterior and posterior components. The anterior pterygoid
arose from the lateral edge and the dorsal surface of the
pterygoid flange. The burred dorsal recurvature of the edge resembles
that of the flange of crocodiles, which serves as part of the
origin of the anterior pterygoid in those animals. In Captorhinus
the attachment of the anterior pterygoid to the edge of the flange
was probably tendinous, judging from the extent of the development
of the edge of the flange. From the edge the origin extended
medially across the dorsal surface of the flange; the ridging of this
surface is indistinct, leading to the supposition that here the origin
was more likely to have been fleshy than tendinous.
The anterior pterygoid extended obliquely backward and downward
from its origin, passed medial to the temporal muscle and
inserted on the ventral and medial surfaces of the splenial and
angular bones beneath the Meckelian fossa. The spatial relationship
between the palate and quadrate-articular joint indicate that
the muscle was probably a minor adductor in Captorhinus.
When the jaw was adducted, the insertion of the anterior pterygoid
was in a plane nearly level with the origin. Contraction of
the anterior pterygoid when the jaw was in this position pulled the
mandible forward and did not adduct it. Maximum depression of
the mandible produced maximum disparity vertically between the
levels of the origin and insertion. The force exerted by the anterior[Pg 665]
pterygoid upon the mandible when fully lowered most nearly approached
the perpendicular to the long axes of the mandibular
rami, and the resultant force acting on the mandible was adductive.
The adductive component of force therefore decreased as the
jaw swung upward, with the result that the anterior pterygoid could
only have been active in initiating adduction and not in sustaining it.
The evidence regarding the position and extent of the posterior
pterygoid is more veiled. On the medial surface of the mandible,
the prearticular and articular bones meet in a ridge that ventrally
rims the glenoid cavity (Fig. 4). The ridge extends anteriorly and
curves slightly in a dorsal direction and meets the Meckelian fossa.
The curved part of the ridge is made of the prearticular bone alone.
A small hollow above the ridge, anterior to the glenoid cavity, faces
the medial plane of the skull and is bordered by the articular bone
behind and above, and by the Meckelian fossa in front.
The surfaces of the hollow and the prearticular-articular ridge
bear tiny grooves and ridges that seem to be muscle scars. The
entire area of the hollow and its bordering features was probably
the area of insertion of the posterior pterygoid.
However, the area of insertion lies mostly ventral to the articulating
surface of the articular bone and extends but slightly in front
of it. Seemingly little lever effect could be exercised by an adductor
attaching in this position, namely, at the level of the fulcrum of the
mandibular ramus.
The posterior pterygoid muscle probably arose from the anterior
portion of the pterygoid wing of the quadrate, from a ridge on the
ventromedial surface. From the relationship of the muscle to the
articulation of the jaw with the skull, it may be deduced that the
muscle was limited in function to the stabilization of the quadrate-articular
joint by keeping the articular surfaces in close contact
with each other and by preventing lateral slipping.
Finally there is evidence for an adductor between the temporal
and masseter masses. The anterior dorsal lip of the Meckelian
fossa supports a small knob to which this muscle attached, much as
in Sphenodon (Romer, 1956:18, Fig. 12). Presumably the muscle
was sheetlike and attached to the skull roof, medial to the attachment
of the masseter.
A pseudotemporal may have been present, but evidence to indicate
its extent and position is lacking. The muscle usually arises
from the epipterygoid and nearby areas of the braincase and skull
roof and inserts in the anterior parts of the fossa of the jaw. In
Captorhinus the lateral wing of the pterygoid cuts across the fossa,[Pg 666]
effectively blocking it from the upper and medial parts of the skull,
the areas of origin for the pseudotemporal.
Dimetrodon
The morphology of the skull of Dimetrodon closely resembles
that of the primitive Haptodus (Haptodontinae, Sphenacodontidae),
and "hence may be rather confidently described as that of
the family as a whole" (Romer and Price, 1940:285). The major
differences between the two genera are in the increased specialization
of the dentition, the shortening of the lacrimal, and the development
of long vertebral spines in Dimetrodon. The absence of gross
differences in the areas of the skull associated with the groups of
muscles with which this study is concerned, implies a similarity
in the patterns of musculature between the two groups. Romer
and Price suggest that Haptodus, although too late in time to be
an actual ancestor, shows "all the common features of the Dimetrodon
group on the one hand and the therapsids on the other." The
adductors of the jaw of Dimetrodon were probably little changed
from those of the Haptodontinae and represent a primitive condition
within the suborder.
Dimetrodon and Captorhinus differ in the bones associated with
the adductor mechanism; the area behind the orbit in Dimetrodon
is relatively shorter, reducing the comparative longitudinal extent
of the adductor chamber. Furthermore, the dermal roof above the
adductor chamber slopes gently downward from behind the orbit
to its contact with the occipital plate in Dimetrodon. Temporal
fenestrae are, of course, present in Dimetrodon.
Musculature
The adductor musculature of the lower jaw in Dimetrodon was
divided into lateral and medial groups (Figs. 5, 6). The lateral
division consisted of temporal and masseter masses. The temporal
arose from the upper rim of the temporal opening, from the lateral
wall of the skull behind the postorbital strut, and from the dorsal
roof of the skull. The bones of origin included jugal, postorbital,
postfrontal, parietal and squamosal. This division may also have
arisen from the fascia covering the temporal opening (Romer and
Price, 1940:53). The muscle passed into the Meckelian fossa of the
mandible and inserted on the angular, surangular, prearticular,
coronoid and dentary bones. Insertion on the lips of the fossa also
probably occurred.
The lateral division arose from the lower rim of the temporal
opening and from the bones beneath. Insertion was in the[Pg 667]
Meckelian fossa and on the dorsal surface of the adjoining coronoid
process.

Fig. 5. Dimetrodon. Internal aspect of skull, showing masseter and
temporal muscles. Skull modified from Romer and Price (1940). Approx. × 1/4.
The reconstruction of the progressively widening masseter as it
traveled to the mandible follows from the progressively widening
depression on the internal wall of the cheek against which the
muscle must have been appressed. The depressed surface included
the posterior wing of the jugal, the whole of the squamosal, and
probably the anteriormost parts of the quadratojugal. Expansion
of the muscle rostrally was prevented by the postorbital strut that
protected the orbit (Romer and Price, 1940:53).
The sphenacodonts possess the primitive rhynchocephalian kind
of palate. In Sphenodon the anterior pterygoid muscle arises from
the dorsal surface of the pterygoid bone and from the adjacent
bones. A similar origin suggests itself for the corresponding muscle,
the second major adductor mass, in Dimetrodon.
From the origin the muscle passed posterodorsad and laterad of
the pterygoid flange. Insertion was in the notch formed by the
reflected lamina of the angular, as suggested by Watson (1948).
In Dimetrodon the relationship of the dorsal surface of the palate
and the ventromedial surface of the mandible in front of the articulation
with the quadrate is unlike that in Captorhinus. When the
mandible of Dimetrodon is at rest (adducted), a line drawn between[Pg 668]
these two areas is oblique, between 30 and 40 degrees from
the horizontal. Depression of the mandible increases this angle.
The insertion of the anterior pterygoid is thus always considerably
below the origin, permitting the muscle to be active throughout
the movement of the mandible, from maximum depression to complete
adduction. This was a major factor in adding substantially
to the speed and power of the bite.
The presence and extent of a posterior pterygoid is more difficult
to assess, because of the closeness of the glenoid cavity and the
raised ridge of the prearticular, and the occupancy of at least part
of this region by the anterior pterygoid. In some specimens of
Dimetrodon the internal process of the articular is double (see
Romer and Price, 1940:87, Fig. 16) indicating that there was a
double insertion here. Whether the double insertion implies the
insertion of two separate muscles is, of course, the problem. Division
of the pterygoid into anterior and posterior portions is the
reptilian pattern (Adams, 1919), and such is adhered to here, with
the posterior pterygoid arising as a thin sheet from the quadrate
wing of the pterygoid and the quadrate, and inserting by means
of a tendon on the internal process of the articular, next to the
insertion of the anterior pterygoid.
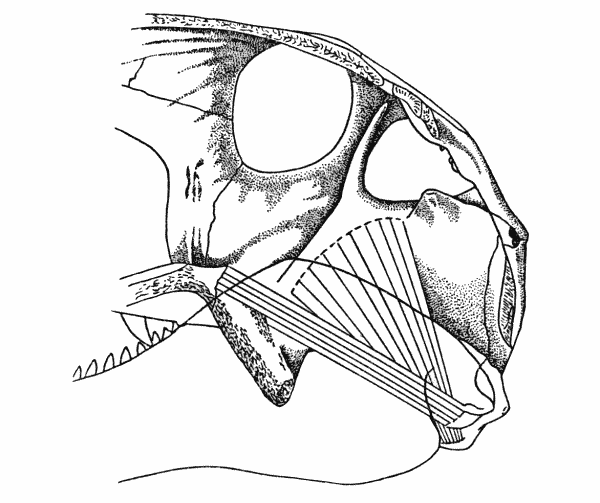
Fig. 6. Dimetrodon. Internal aspect of
right cheek, showing anterior and posterior
pterygoid muscles. Skull modified from
Romer and Price (1940). Approx. × 1/4.
Watson (1948) has reconstructed the musculature of the jaw in
Dimetrodon with results that are at variance with those of the
present study. Watson recognized two divisions, an inner temporal[Pg 669]
and an outer masseteric, of the capitimandibularis, but has pictured
them (830: Fig. 4; 831: Fig. 5C) as both arising from the inner
surface of the skull roof above the temporal opening. But in
Captorhinus the masseter arose from the lower part of the cheek
close to the outer surface of the coronoid process. Watson has
shown (1948:860, Fig. 17B) the same relationship of muscle to
zygoma in Kannemeyeria sp. It is this arrangement that is also
characteristic of mammals and presumably of Thrinaxodon. In
view of the consistency of this pattern, I have reconstructed the
masseter as arising from the lower wall of the cheek beneath the
temporal opening.
Watson's reconstruction shows both the temporal and masseter
muscles as being limited anteroposteriorly to an extent only slightly
greater than the anteroposterior diameter of the temporal opening.
The whole of the posterior half of the adductor chamber is unoccupied.
More probably this area was filled by muscles. The
impress on the inner surface of the cheek is evident, and the extent
of both the coronoid process and Meckelian opening beneath the
rear part of the chamber indicate that muscles passed through this
area.
Watson remarked (1948:829-830) that the Meckelian opening in
Dimetrodon "is very narrow and the jaw cavity is very small. None
the less, it may have been occupied by the muscle or a ligament
connected to it. Such an insertion leaves unexplained the great
dorsal production of the dentary, surangular and coronoid. This
may merely be a device to provide great dorsal-ventral stiffness to
the long jaw, but it is possible and probable that some part of the
temporal muscle was inserted on the inner surface of the coronoid.
Indeed a very well-preserved jaw of D. limbatus? (R. 105: Pl. I,
Fig. 2) bears a special depressed area on the outer surface of the
extreme hinder end of the dentary which differs in surface modelling
from the rest of the surface of the jaw, has a definite limit anteriorly,
and may represent a muscle insertion. The nature of these insertions
suggests that the muscle was already divided into two parts,
an outer masseter and an inner temporalis." But, unaccountably,
Watson's illustration (1948:830, Fig. 4) of his reconstruction limits
the insertion of the temporal to the anterior limit of the Meckelian
opening and a part of the coronoid process above it. No muscle
is shown entering the Meckelian canal. It seems more likely that
the temporal entered and inserted in the canal and on its dorsal
lips. The masseter inserted lateral to it, over the peak of the
coronoid process, and overlapping onto the dorsalmost portions of[Pg 670]
its external face, as Watson has illustrated (Plate I, middle fig.).
I am in agreement with Watson's reconstruction of the origins
for both the anterior and posterior pterygoid muscles. On a functional
basis, however, I would modify slightly Watson's placement
of the insertions of these muscles. Watson believed that the jaw
of Dimetrodon was capable of anteroposterior sliding. The articular
surfaces of the jaws of Dimetrodon that I have examined indicate
that this capability, if present at all, was surely of a very limited
degree, and in no way comparable to that of Captorhinus. The
dentition of Dimetrodon further substantiates the movement of the
jaw in a simple up and down direction. The teeth of Dimetrodon
are clearly stabbing devices; they are not modified at all for grinding
and the correlative freedom of movement of the jaw that that function
requires in an animal such as Edaphosaurus. Nor are they
modified to parallel the teeth of Captorhinus. The latter's diet is
less certain, but presumably it was insectivorous (Romer, 1928).
With the requisite difference in levels of origin and insertion of
the anterior pterygoid in Dimetrodon insuring the application of
force throughout the adduction of the jaws, it would seem that the
whole of the insertion should be shifted downward and outward
in the notch. If this change were made in the reconstruction, the
anterior pterygoid would have to be thought of as having arisen by
a tendon from the ridge that Watson has pictured (1948:828, Fig. 3)
as separating his origins for anterior and posterior pterygoids. The
posterior pterygoid, in turn, arose by tendons from the adjoining
lateral ridge and from the pterygoid process of Romer and Price.
Tendinous origins are indicated by the limitations of space in this
area, by the strength of the ridges pictured and reported by Watson,
and by the massiveness of the pterygoid process of Romer and Price.
Discussion
A comparison of the general pattern of the adductor musculature
of Captorhinus and Dimetrodon reveals an expected similarity. The
evidence indicates that the lateral and medial temporal masses were
present in both genera. The anterior pterygoid aided in initiating
adduction in Captorhinus, whereas in Dimetrodon this muscle was
adductive throughout the swing of the jaw. Evidence for the
presence and extent of a pseudotemporal muscle in both Captorhinus
and Dimetrodon is lacking. The posterior division of the
pterygoid is small in Captorhinus. In Dimetrodon this muscle has
been reconstructed by Watson as a major adductor, an arrangement
that is adhered to here with but slight modification.[Pg 671]
The dentition of Captorhinus suggests that the jaw movement
in feeding was more complex than the simple depression and adduction
that was probably characteristic of Dimetrodon and supports
the osteological evidence for a relatively complex adductor
mechanism.
In Captorhinus the presence of an overlapping premaxillary beak
bearing teeth that are slanted posteriorly requires that the mandible
be drawn back in order to be depressed. Conversely, during
closure, the jaw must be pulled forward to complete full adduction.
The quadrate-articular joint is flat enough to permit such anteroposterior
sliding movements. The relationship of the origin and
insertion of the anterior pterygoid indicates that this muscle, ineffective
in maintaining adduction, may well have acted to pull
the mandible forward, in back of the premaxillary beak, in the last
stages of adduction. Abrasion of the sides of the inner maxillary
and outer dentary teeth indicates that tooth-to-tooth contact did
occur. Whether such abrasion was due to contact in simple vertical
adduction or in anteroposterior sliding is impossible to determine,
but the evidence considered above indicates the latter probability.
Similarities of Protorothyris to sphenacodont pelycosaurs in the
shape of the skull and palate already commented upon by Watson
(1954) and Hotton (1961) suggest that the condition of the adductors
in Dimetrodon is a retention of the primitive reptilian
pattern, with modifications mainly limited to an increase in size
of the temporalis. Captorhinus, however, seems to have departed
rather radically from the primitive pattern, developing specializations
of the adductors that are correlated with the flattening of the
skull, the peculiar marginal and anterior dentition, the modifications
of the quadrate-articular joint, and the development of the coronoid
process.
Thrinaxodon
The evidence for the position and extent of the external adductors
of the lower jaw in Thrinaxodon was secured in part from dissections
of Didelphis marsupialis, the Virginia opossum. Moreover,
comparison of the two genera reveals striking similarities in the
shape and spatial relationships of the external adductors. These
are compared below in some detail.
The sagittal crest in Thrinaxodon is present but low. It arises
immediately in front of the pineal foramen from the confluence of
bilateral ridges that extend posteriorly and medially from the base
of the postorbital bars. The crest diverges around the foramen,[Pg 672]
reunites immediately behind it, and continues posteriorly to its
junction with the supraoccipital crest (Estes, 1961).
In Didelphis the sagittal crest is high and dorsally convex in
lateral aspect, arising posterior to and medial to the orbits, reaching
its greatest height near the midpoint, and sloping down to its termination
at the supraoccipital crest. Two low ridges extend posteriorly
from the postorbital process to the anterior end of the sagittal
crest and correspond to ridges in similar position in Thrinaxodon.
The supraoccipital crest flares upward to a considerable extent
in Thrinaxodon and slopes posteriorly from the skull-roof proper.
The crest extends on either side downward to its confluence with
the zygomatic bar. The area of the crest that is associated with
the temporal musculature is similarly shaped in Didelphis.
The zygomatic bar in each genus is stout, laterally compressed,
and dorsally convex on both upper and lower margins. At the back
of the orbit of Thrinaxodon, the postorbital process of the jugal
extends posterodorsally. At this position in Didelphis, there is but
a minor upward curvature of the margin of the bar.
In Thrinaxodon the dorsal and ventral postorbital processes, arising
from the postorbital and jugal bones respectively, nearly meet
but remain separate. The orbit is not completely walled off from
the adductor chamber. The corresponding processes in Didelphis
are rudimentary so that the confluence of the orbit and the adductor
chamber is complete.
The adductor chamber dorsally occupies slightly less than half
of the total length of the skull of Thrinaxodon; in Didelphis the
dorsal length of the chamber is approximately half of the total
length of the skull.

Fig. 7. Thrinaxodon. Showing masseter and temporal muscles.
Skull after Romer (1956). Approx. × 7/10.
The coronoid process in Thrinaxodon sweeps upward posterodorsally
at an angle oblique to the long axis of the ramus. Angular,
surangular and articular bones extend backward beneath and[Pg 673]
medial to the process. The process extends above the most dorsal
point of the zygomatic bar, as in Didelphis. The mandibular ramus
is ventrally convex in both genera.
The relationships described above suggest that Thrinaxodon and
the therapsids having similar morphology in the posterior region
of the skull possessed a temporal adductor mass that was split into
major medial and lateral components (Fig. 7). The more lateral of
these, the masseter, arose from the inner surface and lower margin
of the zygomatic bar and inserted on the lateral surface of the coronoid
process.
The medial division or temporal arose from the sagittal crest and
supraoccipital crest and the intervening dermal roof. The muscle
inserted on the inner and outer surfaces of the coronoid process
and possibly on the bones beneath.
Thrinaxodon represents an advance beyond Dimetrodon in several
respects. The zygomatic bar in Thrinaxodon extends relatively
far forward, is bowed outward and dorsally arched. Consequently,
the masseter was able to extend from an anterodorsal origin to a
posterior and ventral insertion. The curvature of the jaw transforms
the anterodorsal pull of the muscle into a dorsally directed
adductive movement regardless of the initial angle of the jaw. This
is the generalized mammalian condition.
With the development of the secondary palate the area previously
available for the origin of large anterior pterygoid muscles was
reduced. The development of the masseter extending posteroventrally
from an anterior origin presumably paralleled the reduction
of the anterior pterygoids. The therapsid masseter, as an
external muscle unhindered by the crowding of surrounding organs,
was readily available for the many modifications that have been
achieved among the mammals.
In the course of synapsid evolution leading to mammals, the
temporal presumably became the main muscle mass acting in adduction
of the lower jaw. Its primacy is reflected in the phyletic
expansion of the temporal openings to permit greater freedom of
the muscles during contraction. In the synapsids that lead to mammals,
there is no similar change in the region of the palate that can
be ascribed to the effect of the pterygoid musculature, even though
these adductors, like the temporal, primitively were subjected to
severe limitations of space.
Didelphis
Dissections reveal the following relationships of the external adductors
of the jaw in Didelphis marsupialis (Fig. 8).[Pg 674]
1. Masseter
Origin: ventral surface of zygomatic arch.
Insertion: posteroventral and lateroventral surface of mandible.
2. External temporalis
Origin: sagittal crest; anteriorly with internal temporalis from frontal
bone; posteriorly with internal temporalis from interparietal bone.
Insertion: lateral surface of coronoid process of mandible.
3. Internal temporalis
Origin: sagittal crest and skull roof, including posterior two-thirds of
frontal bone, whole of parietal, and dorsalmost portions of squamosal
and alisphenoid.
Insertion: medial surface of coronoid process; dorsal edge of coronoid
process.

Fig. 8. Didelphis marsupialis. Showing masseter and
temporal muscles. Skull KU 3780, 1 mi. N Lawrence,
Douglas Co., Kansas. × 3/5.
Temporal Openings
In discussions of the morphology and functions of the adductor
mechanism of the lower jaw, the problem of accounting for the
appearance of temporal openings in the skull is often encountered.
Two patterns of explanation have evolved. The first has been the
attempt to ascribe to the constant action of the same selective force
the openings from their inception in primitive members of a
phyletic line to their fullest expression in terminal members. According
to this theory, for example, the synapsid opening appeared
originally to allow freer expansion of the adductor muscles of the
jaw during contraction, and continued selection for that character
caused the openings to expand until the ultimately derived therapsid
or mammalian condition was achieved.
The second course has been the attempt to explain the appearance
of temporal openings in whatever line in which they occurred
by the action of the same constant selective force. According to
the reasoning of this theory, temporal fenestration in all groups was[Pg 675]
due to the need to decrease the total weight of the skull, and
selection in all those groups where temporal fenestration occurs
was to further that end.
Both of these routes of inquiry are inadequate. If modern views
of selection are applied to the problem of explaining the appearance
of temporal fenestrae, the possibility cannot be ignored that:
1. Selective pressures causing the inception of temporal fenestrae differed
from those causing the continued expansion of the fenestrae.
2. The selective pressures both for the inception and continued expansion
of the fenestrae differed from group to group.
3. Selection perhaps involved multiple pressures operating concurrently.
4. Because of different genotypes the potential of the temporal region to
respond to selective demands varied from group to group.
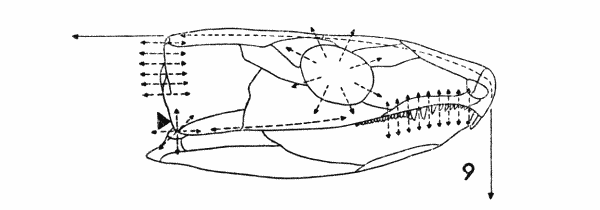
Fig. 9. Captorhinus. Diagram, showing
some hypothetical lines of stress. Approx. × 1.

Fig. 10. Captorhinus. Diagram,
showing areas of internal thickening. Approx. × 1.
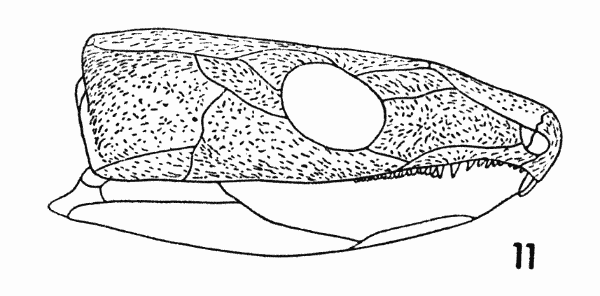
Fig. 11. Captorhinus. Diagram,
showing orientation of sculpture. Approx. × 1.
Secondly, the vectors of mechanical force associated with the
temporal region are complex (Fig. 9). Presumably it was toward
a more efficient mechanism to withstand these that selection on the
cheek region was operating. The simpler and more readily analyzed
of these forces are:
1. The force exerted by the weight of the skull anterior to the cheek and
the distribution of that weight depending upon, for example, the length of the
snout in relation to its width, and the density of the bone.
2. The weight of the jaw pulling down on the suspensorium when the jaw
is at rest and the compression against the suspensorium when the jaw is adducted;
the distribution of these stresses depending upon the length and
breadth of the snout, the rigidity of the anterior symphysis, and the extent of
the quadrate-articular joint.[Pg 676]
3. The magnitude and extent of the vectors of force transmitted through the
occiput from the articulation with the vertebral column and from the pull of
the axial musculature.
4. The downward pull on the skull-roof by the adductor muscles of the
mandible.
5. The lateral push exerted against the cheek by the expansion of the
mandibular adductors during contraction.
6. The necessity to compensate for the weakness in the skull caused by the
orbits, particularly in those kinds of primitive tetrapods in which the orbits
are large.
The distribution of these stresses is further complicated and
modified by such factors as:
1. The completeness or incompleteness of the occiput and the location and
extent of its attachment to the dermal roof.
2. The size and rigidity of the braincase and palate, and the extent and
rigidity of their contact with the skull.
The stresses applied to the cheek fall into two groups. The first
includes all of those stresses that ran through and parallel to the
plane of the cheek initially. The weight of the jaw and snout, the
pull of the axial musculature, and the necessity to provide firm
anchorage for the teeth created stresses that acted in this manner.
The second group comprises those stresses that were applied
initially at an oblique angle to the cheek and not parallel to its
plane. Within this group are the stresses created by the adductors
of the jaw, pulling down and medially from the roof, and sometimes,
during contraction, pushing out against the cheek.
It is reasonable to assume that the vectors of these stresses were
concentrated at the loci of their origin. For example, the effect of
the forces created by the articulation of the jaw upon the skull
was concentrated at the joint between the quadrate, quadratojugal,
and squamosal bones. From this relatively restricted area, the
stresses radiated out over the temporal region. Similarly, the
stresses transmitted by the occiput radiated over the cheek from
the points of articulation of the dermal roof with the occipital
plate. In both of these examples, the vectors paralleled the plane
of the cheek bones. Similar radiation from a restricted area, but
of a secondary nature, resulted from stresses applied obliquely to
the plane of the cheek. The initial stresses caused by the adductors
of the jaw resulted from muscles pulling away from the skull-roof;
secondary stresses, created at the origins of these muscles, radiated
out over the cheek, parallel to its plane.
The result of the summation of all of those vectors was a complex
grid of intersecting lines of force passing in many directions both[Pg 677]
parallel to the plane of the cheek and at the perpendicular or at an
angle oblique to the perpendicular to the plane of the cheek.
Complexities are infused into this analysis with the division of
relatively undifferentiated muscles into subordinate groups. The
differentiation of the muscles was related to changing food habits,
increased mobility of the head, and increase in the freedom of movement
of the shoulder girdle and forelimbs (Olson, 1961:214). As
Olson has pointed out, this further localized the stresses to which
the bone was subjected. Additional localization of stresses was
created with the origin and development of tetrapods (reptiles)
that were independent of an aquatic environment and were subjected
to greater effects of gravity and loss of bouyancy in the
migration from the aqueous environment to the environment of air.
The localization of these stresses was in the border area of the
cheek, away from its center.
What evidence is available to support this analysis of hypothetical
forces transmitted through the fully-roofed skull of such
an animal as Captorhinus?
It is axiomatic that bones or parts of bones that are subject to
increased stress become thicker, at least in part. This occurs
ontogenetically, and it occurs phylogenetically through selection.
Weak bones will not be selected for. Figure 10 illustrates the pattern
of the areas of the skull-roof in the temporal region that are
marked on the internal surface by broad, low thickened ridges.
The position of these ridges correlates well with the position of the
oriented stresses that were presumably applied to the skull of
Captorhinus during life. It can be seen from Figure 10 that the
central area of the cheek is thinner than parts of the cheek that
border the central area. The thickened border areas were the
regions of the cheek that were subjected to greater stress than the
thin central areas.
External evidence of stress may also be present. The pattern of
sculpturing of Captorhinus is presented in Figure 11. The longer
ridges are arranged in a definite pattern. Their position and direction
correlates well with the thickened border of the cheek, the
region in which the stresses are distinctly oriented. For example,
a ridge is present on the internal surface of the squamosal along its
dorsal border. Externally, the sculptured ridges are long and
roughly parallel, both to each other and to the internal ridge.
The central area of the cheek is characterized by a reticulate pattern
of short ridges, without apparent orientation. The thinness
of the bone in this area indicates that stresses were less severe here.[Pg 678]
The random pattern of the sculpture also indicates that the stresses
passed in many directions, parallel to the plane of the cheek and
obliquely to that plane.
Possible Explanation for the Appearance of Temporal Openings
Bone has three primary functions: support, protection and participation
in calcium metabolism. Let us assume that the requirements
of calcium metabolism affect the mass of bone that is selected
for, but do not grossly affect the morphology of the bones
of that mass. Then selection operates to meet the needs for
support within the limits that are set by the necessity to provide
the protection for vital organs. After the needs for protection are
satisfied, the remaining variable and the one most effective in
determining the morphology of bones is selection for increased
efficiency in meeting stress.
Let us also assume that bone increases in size and/or compactness
in response to selection for meeting demands of increased stress,
but is selected against when requirements for support are reduced
or absent. Selection against bone could only be effective within
the limits prescribed by the requirements for protection and calcium
metabolism.
We may therefore assume that there is conservation in selection
against characters having multiple functions. Since bone is an
organ system that plays a multiple role in the vertebrate organism,
a change in the selective pressures that affect one of the roles of
bone can only be effective within the limits set by the other roles.
For example, selection against bone that is no longer essential for
support can occur only so long as the metabolic and protective
needs of the organism provided by that character are not compromised.
If a character no longer has a positive survival value and is
not linked with a character that does have a positive survival value,
then the metabolic demands for the development and maintenance
of that character no longer have a positive survival value. A useless
burden of metabolic demands is placed upon the organism because
the character no longer aids the survival of the organism. If selection
caused, for example, muscles to migrate away from the center
of the cheek, the bone that had previously provided support for
these muscles would have lost one of its functions. If in a population
of such individuals, variation in the thickness of the bone of
the cheek occurred, those with thinner bone in the cheek would be
selected for, because less metabolic activity was diverted to building
and maintaining what is now a character of reduced functional[Pg 679]
significance. A continuation of the process would eliminate the
bone or part of the bone in question while increasing the metabolic
efficiency of the organism. The bone is no longer essential for
support, the contribution of the mass of bone to calcium metabolism
and the contribution of this part of the skeleton to protection have
not been compromised, and the available energy can be diverted
to other needs.
The study of Captorhinus has indicated that the central area of
the cheek was subjected to less stress than the border areas. A
similar condition in basal reptiles may well have been present. A
continued trend in reducing the thickness of the bone of the cheek
in the manner described above may well have resulted in the appearance
of the first reptiles with temporal fenestrae arising from
the basal stock.
Such an explanation adequately accounts for an increased selective
advantage in the step-by-step thinning of the cheek-wall prior
to the time of actual breakthrough. It is difficult to see the advantage
during such stages if explanations of weight reduction or
bulging musculature are accepted.
After the appearance of temporal fenestrae, selection for the
classical factors is quite acceptable to explain the further development
of fenestration. The continued enlargement of the temporal
fenestrae in the pelycosaur-therapsid lineage undoubtedly was
correlated with the advantages accrued from securing greater space
to allow increased lateral expansion of contracting mandibular adductors.
Similarly, weight in absolute terms can reasonably be
suggested to explain the dramatic fenestration in the skeletons of
many large dinosaurs.
Literature Cited
Adams, L. A.
1919. Memoir on the phylogeny of the jaw muscles in recent and fossil
vertebrates. Annals N. Y. Acad. Sci., 28:51-166, 8 pls.
Estes, R.
1961. Cranial anatomy of the cynodont reptile Thrinaxodon liorhinus.
Bull. Mus. Comp. Zool., 125(6):165-180, 4 figs., 2 pls.
Hotton, N.
1960. The chorda tympani and middle ear as guides to origin and development
of reptiles. Evolution, 14(2):194-211, 4 figs.
Olson, E. C.
1961. Jaw mechanisms: rhipidistians, amphibians, reptiles. Am. Zoologist,
1(2):205-215, 7 figs.
Romer, A. S.
1928. Vertebrate faunal horizons in the Texas Permo-Carboniferous redbeds.
Univ. Texas Bull., 2801:67-108, 7 figs.
1956. Osteology of the reptiles. Univ. Chicago Press, xxii + 772 pp.,
248 figs.[Pg 680]
Romer, A. S. and Price, L. I.
1940. Review of the Pelycosauria. Geol. Soc. Amer. Special Papers, No.
28, x + 538 pp., 71 figs., 46 pls.
Watson, D. M. S.
1948. Dicynodon and its allies. Proc. Zool. Soc. London, 118:823-877,
20 figs., 1 pl.
1954. On Bolosaurus and the origin and classification of reptiles. Bull.
Mus. Comp. Zool., 111(9):200-449, 37 figs.
Transmitted December 5, 1963.
30-1522
Featured Books

Systematic Status of the Colubrid Snake, Leptodeira discolor Günther
William Edward Duellman
ollected inOaxaca, México, by Auguste Sallé. Information concerning thescutellation and coloration...

Bee Hunting: A Book of Valuable Information for Bee Hunters
John Ready Lockard
onder that after forty years of undiminished passion for sports of this kind that I can truthfully s...

Seaside Studies in Natural History. Marine Animals of Massachusetts Bay. Radiates.
Elizabeth Cabot Cary Agassiz and Alexander Agassiz
the marine animalscommon to our shores. There are many English books of this kind; but theyrelate c...

The People of the Mist
H. Rider Haggard
T ARGUMENT XXXV. BE NOBLE OR BE BASE XXXVI. HOW OTTER CAME BACK XXXVII. “I AM REPAID, QUEEN” XXX...

Scaramouche: A Romance of the French Revolution
Rafael Sabatini
NT LE CHAPELIER CHAPTER IV. AT MEUDON CHAPTER V. MADAME DE PLOUGAST...

The Iron Horse
R. M. Ballantyne
that large hairy individual came in of an evening, and, catching her in his long arms, pressed her ...

Stories by English Authors: Germany (Selected by Scribners)
asked the lady, sympathetically. “No,” said the young girl; “I had none to lose.” A...

Short Stories of Various Types
he author and his relation to the thought of his time, critical opinions of the work in question cho...
Browse by Category
Join Our Literary Community
Subscribe to our newsletter for exclusive book recommendations, author interviews, and upcoming releases.
Comments on "The Adductor Muscles of the Jaw In Some Primitive Reptiles" :